Correct answer is (D)
CP CV = R for ideal gas and gas behaves as ideal gas at high temperature, so TP > TQ
11. (JEE Main 2021 (Online) 25th July Morning Shift )
For a gas CP CV = R in a state P and CP CV = 1.10 R in a state Q, TP and TQ are the temperatures in two different states P and Q respectively. Then
(A) TP = TQ
(B) TP < TQ
(C) TP = 0.9 TQ
(D) TP > TQ
Correct answer is (D)
CP CV = R for ideal gas and gas behaves as ideal gas at high temperature, so TP > TQ
12. (JEE Main 2021 (Online) 25th July Morning Shift )
Two different metal bodies A and B of equal mass are heated at a uniform rate under similar conditions.
The
variation of temperature of the bodies is graphically represented as shown in the figure. The ratio of
specific heat capacities is :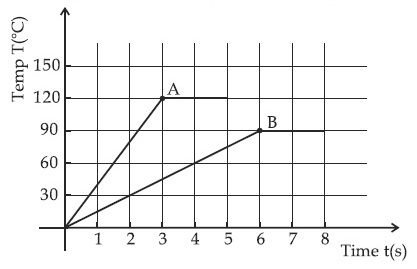
(A)
(B)
(C)
(D)
Correct answer is (B)
13. (JEE Main 2021 (Online) 20th July Evening Shift )
The correct relation between the degrees of freedom f and the ratio of specific heat is :
(A)
(B)
(C)
(D)
Correct answer is (A)
14. (JEE Main 2021 (Online) 17th March Evening Shift )
If one mole of the polyatomic gas is having two vibrational modes and is the ratio of molar specific heats for polyatomic gas then the value of is :
(A) 1.02
(B) 1.35
(C) 1.2
(D) 1.25
Correct answer is (C)
For polyatomic gas molecule has 3 rotational degrees of freedom,
3 translational degrees of freedom, and 2 vibrational modes.
So, number of vibrational degrees of freedom = 2
2 = 4
Degree of freedom of polyatomic gas
f = T + R + V
f = 3 + 3 + 4 =
10
15. (JEE Main 2021 (Online) 17th March Morning Shift )
A polyatomic ideal gas has 24 vibrational modes. What is the value of ?
(A) 1.37
(B) 1.30
(C) 1.03
(D) 10.3
Correct answer is (C)
f = 3T + 3R + 24V
=
30
= 1 +
= 1 +
= 1.066
Nearest Ans. = 1.03