Correct answer is (A)
The net work in the cycle
is
36. (AIEEE 2009 )
Two moles of helium gas are taken over the cycle
, as shown in the
- diagram.
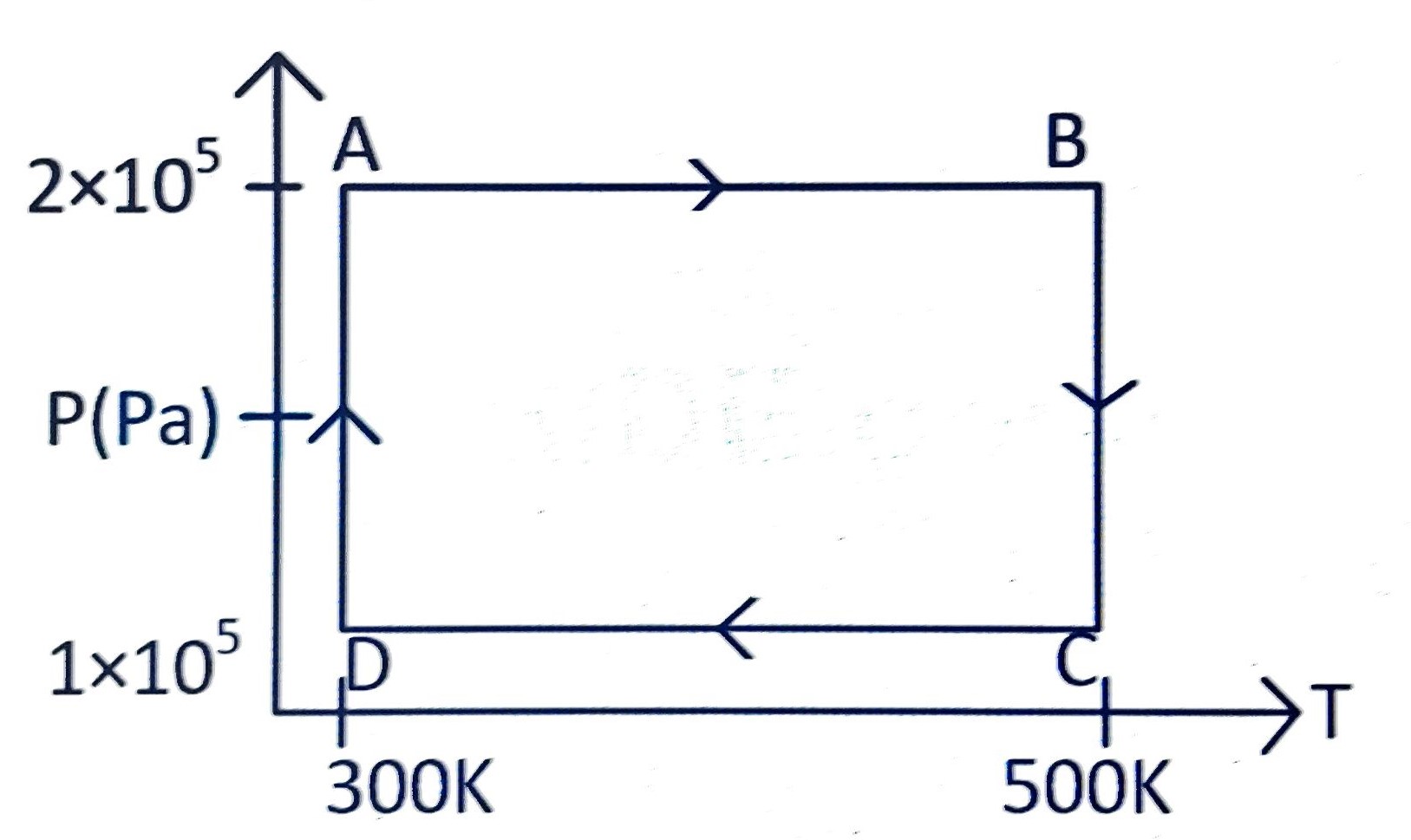
The net work done on the gas in the cycle is:
(A)
(B)
(C)
(D) zero
Correct answer is (A)
The net work in the cycle
is
37. (AIEEE 2007 )
When a system is taken from state
to state
along the path iaf, it is found that
cal and
. Along the path
along the path
is
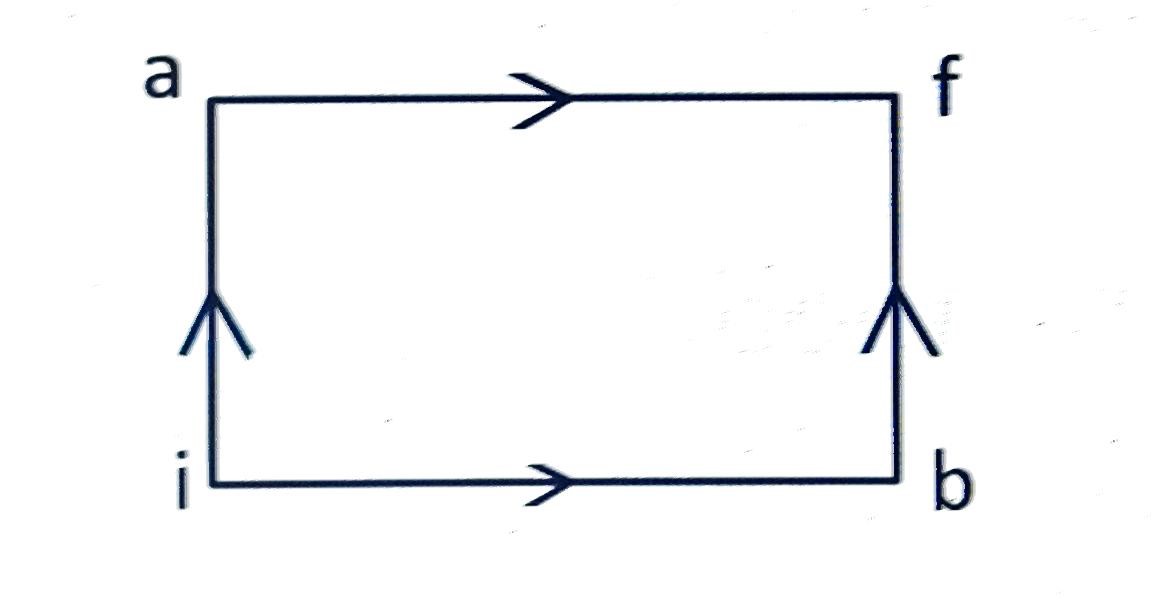
(A)
(B)
(C)
(D)
Correct answer is (B)
For path iaf,
For path ibf,
38. (AIEEE 2006 )
Two rigid boxes containing different ideal gases are placed on a table. Box A contains one mole of nitrogen at temperature while Box contains one mole of helium at temperature The boxes are then put into thermal contact with each other, and heat flows between them until the gases reach a common final temperature (ignore the heat capacity of boxes). Then, the final temperature of the gases, in terms of is
(A)
(B)
(C)
(D)
Correct answer is (C)
Heat lost by He
Heat gained by
39. (AIEEE 2005 )
A system goes from
to
via two processes
and
as shown in figure. If
and
are the changes in internal energies in the processes
and
respectively, then
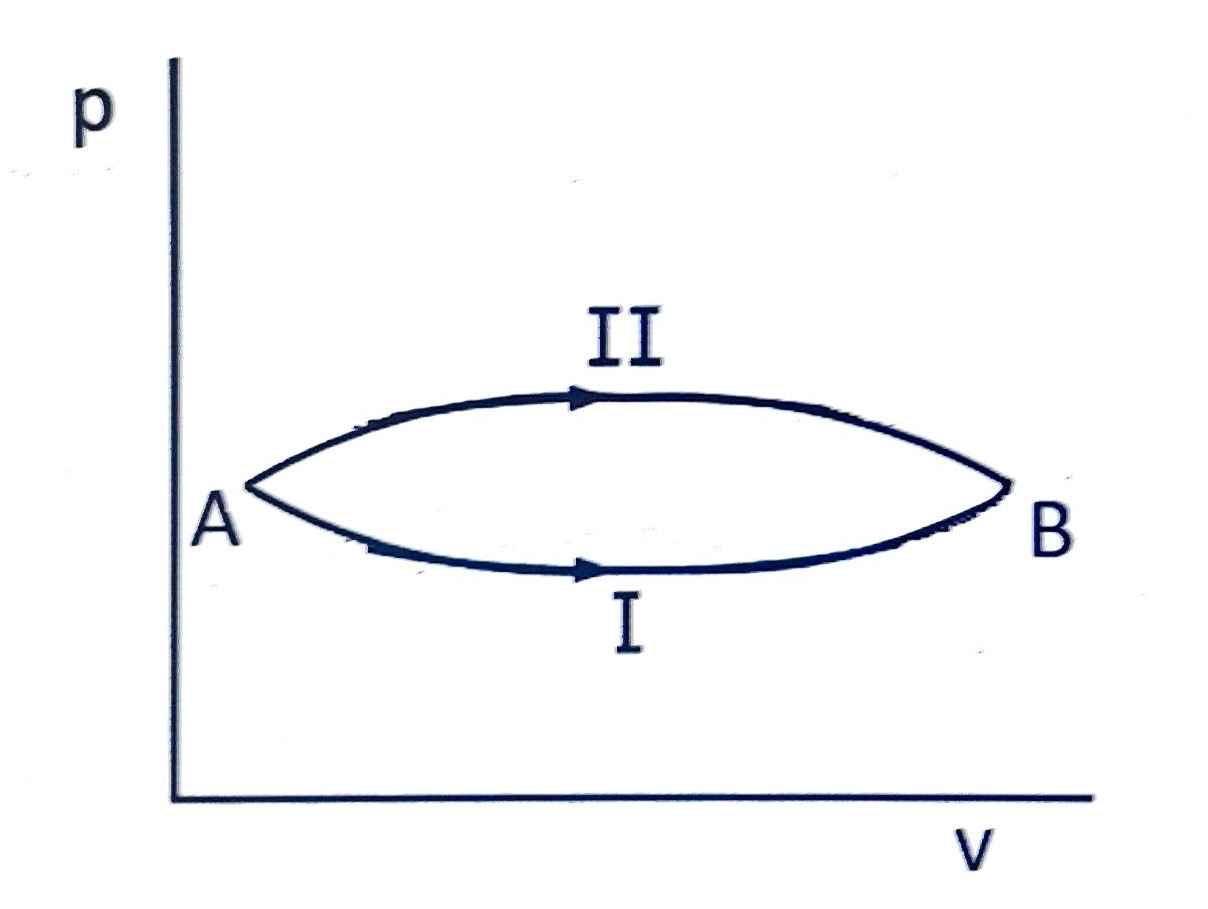
(A) relation between and can not be determined
(B)
(C)
(D)
Correct answer is (B)
Change in internal energy do not depend upon the path followed by the process. It only depends on initial and final states
40. (AIEEE 2003 )
Which of the following parameters does not characterize the thermodynamic state of mattter?
(A) Temperature
(B) Pressure
(C) Work
(D) Volume
Correct answer is (C)
Work is a path function. The remaining three parameters are state function.