Correct Option is (D)
Phenols show weak acidic character while alcohols are neutral. Electron-withdrawing groups (like ) increase the acidity of substituted phenols. Hence, p-nitrophenol has the highest acidic strength among the given compound.
1. ⇒ (MHT CET 2023 10th May Morning Shift )
Identify the compound with highest acidic strength from following.
A. Ethanol
B. t-Butyl alcohol
C. Phenol
D. p-Nitrophenol
Correct Option is (D)
Phenols show weak acidic character while alcohols are neutral. Electron-withdrawing groups (like ) increase the acidity of substituted phenols. Hence, p-nitrophenol has the highest acidic strength among the given compound.
2. ⇒ (MHT CET 2023 10th May Morning Shift )
Which among the following compounds reacts fastly with ?
A.
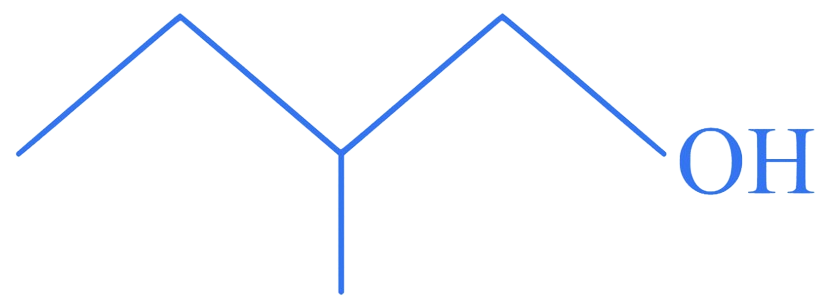
B.
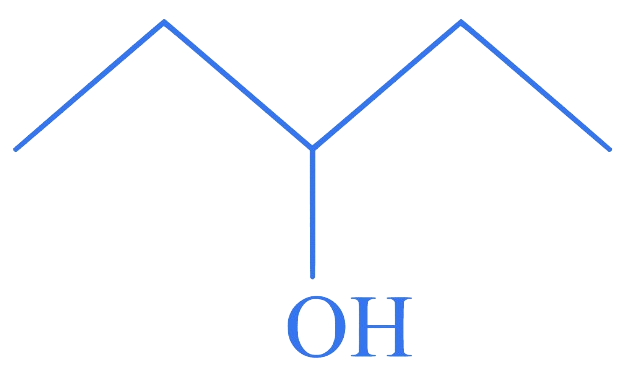
C.
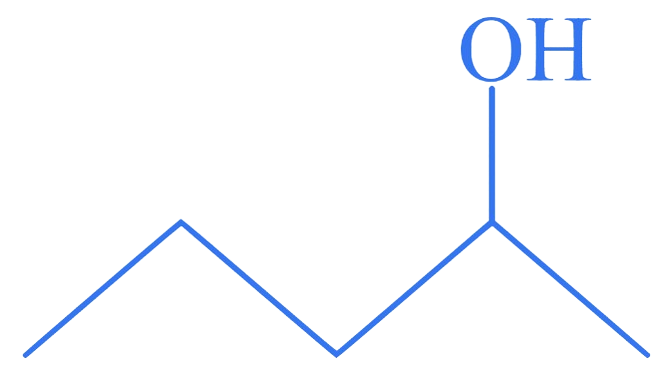
D.
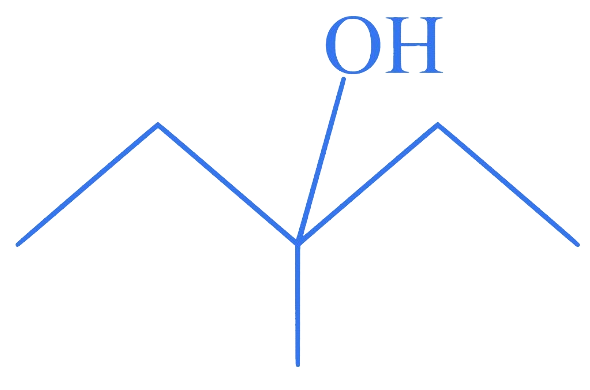
Correct Option is (D)
The order of reactivity of alcohols with a given haloacid is .
Hence, compound (D), a tertiary alcohol, will react fastly with .
3. ⇒ (MHT CET 2023 9th May Evening Shift )
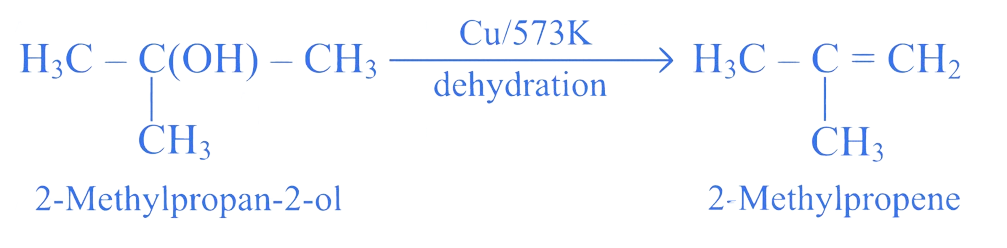
Identify the product formed when vapours of 2-methylpropan-2-ol are passed over hot copper.
A. Propanone
B. 2-Methylpropene
C. 2-Methylpropanoic acid
D. Propanal
Correct Option is (B)
4. ⇒ (MHT CET 2023 9th May Evening Shift )
Which among the following reactions occurs by breaking of bond in alcohol?
A. Reaction with propionic acid.
B. Reaction with acetic anhydride
C. Reaction with phosphorus trichloride
D. Reaction with acetyl chloride
Correct Option is (C)
5. ⇒ (MHT CET 2023 9th May Morning Shift )
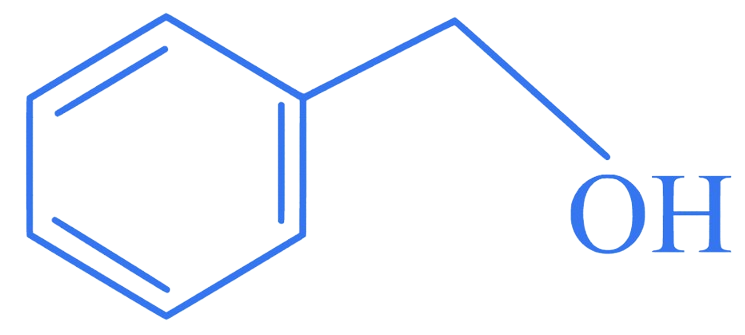
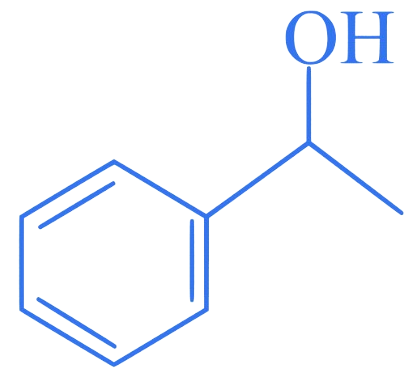
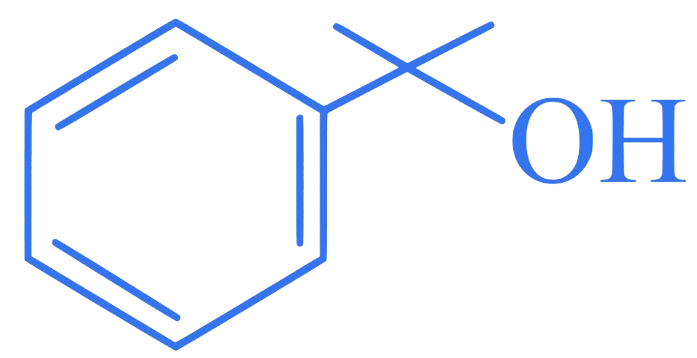
Which of the following is secondary benzylic alcohol?
A.
B.
C.
D.
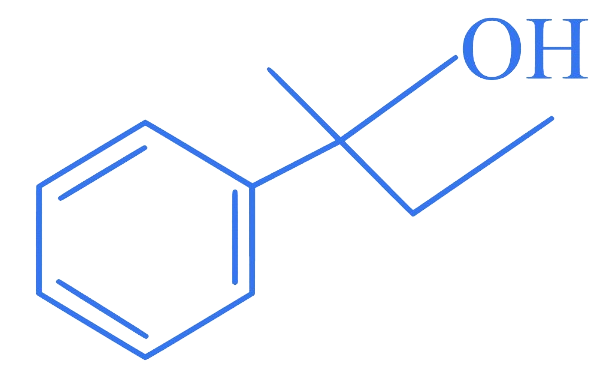
Correct Option is (B)
Currently no explanation available