Correct Option is (C)
in has less than eight electrons in its valence shell. It has only four electrons. Hence, it has an incomplete octet. Molecules like have an expanded octet. They have more than eight electrons around the central atom.
1. ⇒ (MHT CET 2023 11th May Morning Shift )
Identify a molecule with incomplete octet from following.
A.
B.
C.
D.
Correct Option is (C)
in has less than eight electrons in its valence shell. It has only four electrons. Hence, it has an incomplete octet. Molecules like have an expanded octet. They have more than eight electrons around the central atom.
2. ⇒ (MHT CET 2023 10th May Evening Shift )
What is the number of unpaired electrons in molecule?
A. 0
B. 1
C. 2
D. 3
Correct Option is (B)
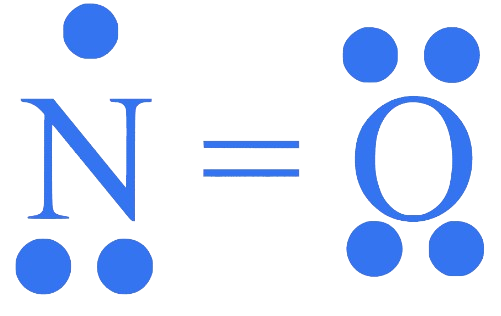
No. of unpaired electrons = 1