Correct Option is (B)
Formation of [CoF]
It is an example of spd hybridization. An octahedral complex shows paramagnetic behaviour. It is therefore called outer orbital or high spin or spin free complex.
Orbitals of Co ion
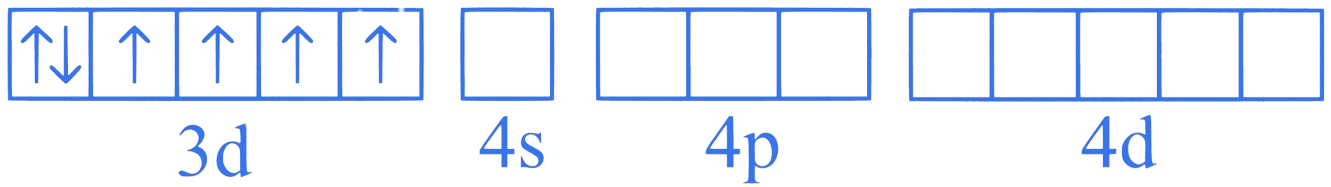
Since F is a weak ligand, there is no spin pairing effect and Co possesses 4 unpaired electrons.
Co undergoing spd hybridization
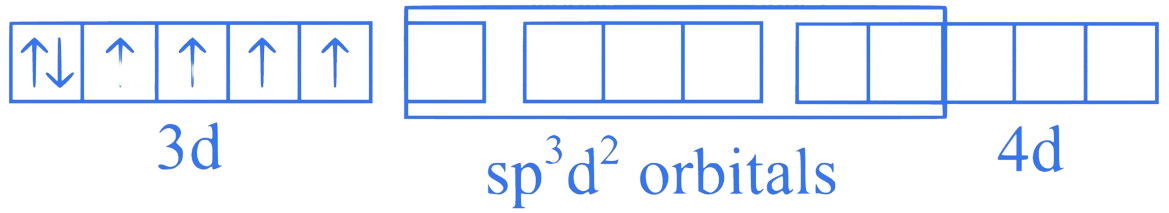
[CoF] (Outer orbital or High spin complex)
