Correct Option is (B)
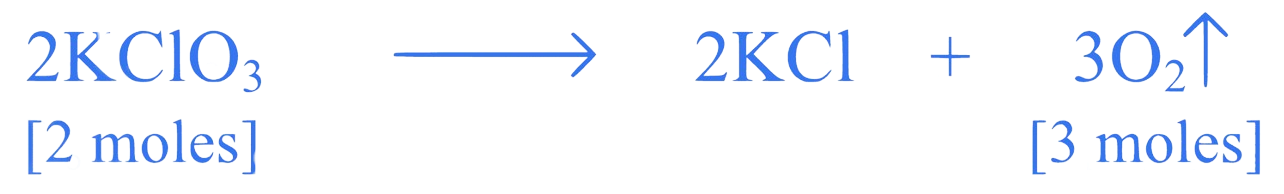
2 moles of
3 moles of at STP occupy
Thus, of potassium chlorate will liberate of oxygen gas.
Let '' gram of liberate of oxygen gas at S.T.P.
1. ⇒ (MHT CET 2023 12th May Evening Shift )
What is the mass of required to liberate 22. oxygen at STP during thermal decomposition?
Molar Mass of
A. 122.5 g
B. 81.67 g
C. 10.25 g
D. 8.16 g
Correct Option is (B)
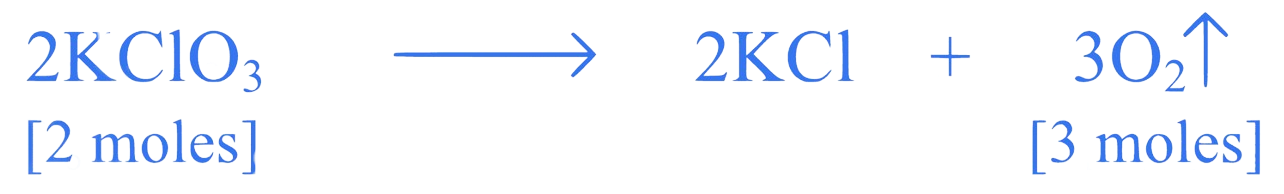
2 moles of
3 moles of at STP occupy
Thus, of potassium chlorate will liberate of oxygen gas.
Let '' gram of liberate of oxygen gas at S.T.P.
2. ⇒ (MHT CET 2023 12th May Morning Shift )
What volume of at STP is obtained by complete combustion of carbon?
A.
B.
C.
D.
Correct Option is (B)
3. ⇒ (MHT CET 2023 11th May Morning Shift )
What is the volume in occupied by of ammonia gas at STP?
A. 2.24
B. 22.4
C. 56.0
D. 67.2
Correct Option is (D)
4. ⇒ (MHT CET 2021 21th September Evening Shift )
What is the volume occupied by 16 g methane gas at STP?
A. 1140 cm
B. 22400 cm
C. 214 cm
D. 12.4 cm
Correct Option is (B)
1 mole of CH = 16 g of CH = 22400 cm at STP