Correct Option is (A)
The integrated rate law for the first order reaction is
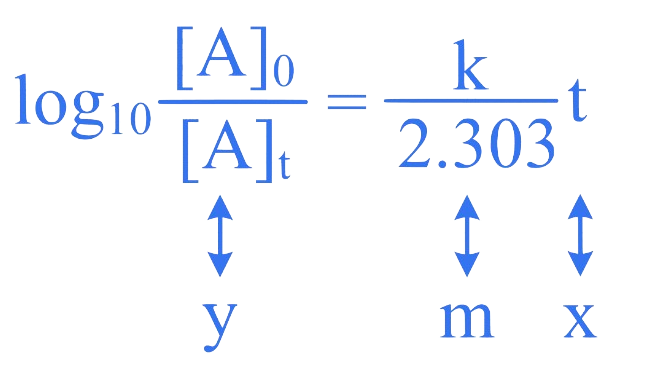
The graph of versus time is a straight line passing through origin with slope .
1. ⇒ (MHT CET 2023 11th May Morning Shift )
Slope of the graph between (y axis) and time ( axis) for first order reaction is equal to:
A.
B.
C.
D.
Correct Option is (A)
The integrated rate law for the first order reaction is
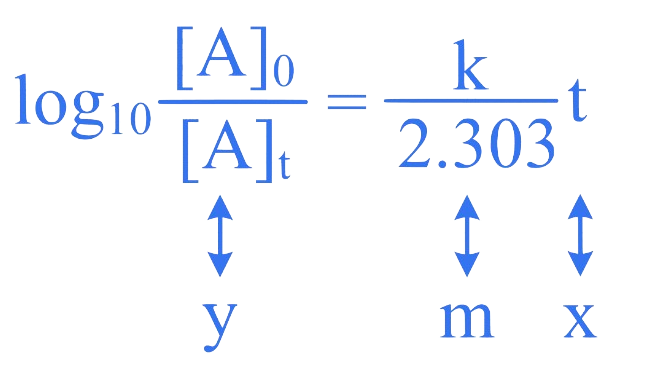
The graph of versus time is a straight line passing through origin with slope .
2. ⇒ (MHT CET 2023 9th May Morning Shift )
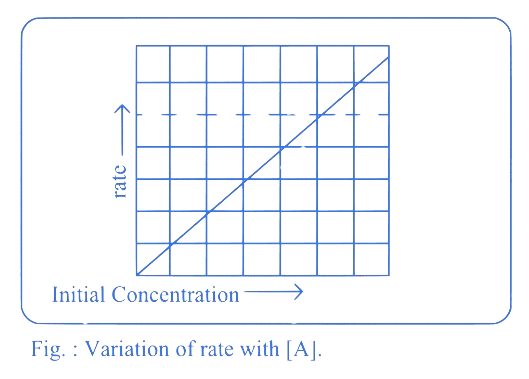
Which from following is the slope of the graph of rate versus concentration of the reactant for first order reaction?
A.
B.
C.
D.
Correct Option is (B)
For a first-order reaction, the rate of the reaction is directly proportional to the concentration of the reactant. The rate law for a first-order reaction can be written as :
where is the rate constant.
The graph of rate versus concentration of the reactant for a first-order reaction would be a straight line with a slope equal to the rate constant . This is because the rate is directly proportional to the concentration, making the slope of the line the rate constant itself.
Therefore, the correct answer is :
Option B :
3. ⇒ (MHT CET 2021 21th September Morning Shift )
Slope of the graph between rate ( -axis) and -axis) for the first order reaction is equal to
A.
B.
C.
D.
Correct Option is (A)
A plot of rate versus [A] is a straight line passing through origin. This is shown in Fig. The slope of straight line = k.