Explanation
Correct answer is D
The reaction is
At the anode (Oxidation):
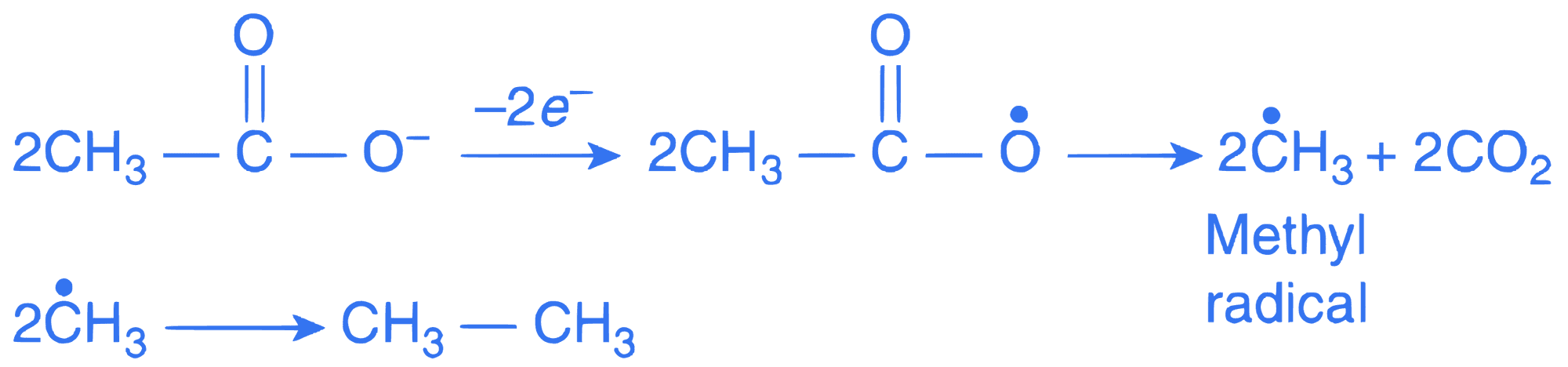
At the cathode (Reduction):
Total number of moles of gases
= moles of C2H6 + moles of CO2 + moles of H2
L
20. ⇒ (JEE Main 2016 (Online) 10th April Morning Slot )
Oxidation of succinate ion produces ethylene and carbon dioxide gases. On passing 0.2 Faraday electricity through an aqueous solution of potassium succinate, the total volume of gases (at both cathode and anode) at STP (1 atm and 273 K) is :
(A) 2.24 L
(B) 4.48 L
(C) 6.72 L
(D) 8.96 L
Explanation
Correct answer is D
The reaction is
At the anode (Oxidation):

At the cathode (Reduction):
Total number of moles of gases
= moles of C2H6 + moles of CO2 + moles of H2
L
21. ⇒ (JEE Main 2015 (Offline) )
Two Faraday of electricity is passed through a solution of CuSO4. The mass of copper deposited at the cathode is: (at. mass of Cu = 63.5 amu)
(A) 63.5 g
(B) 2 g
(C) 127 g
(D) 0 g
Explanation
Correct answer is A
deposit mol
22. ⇒ (AIEEE 2005)
Aluminium oxide may be electrolysed at 1000oC to furnish aluminium metal (Atomic mass = 27 amu; 1 Faraday = 96,500 Coulombs). The cathode reaction is Al3+ + 3e- Alo To prepare 5.12 kg of aluminium metal by this method would require
A. 5.49 107 C of electricity
B. 1.83 107 C of electricity
C. 5.49 104 C of electricity
D. 5.49 101 C of electricity
Correct Answer is Option (A)
mole of
of is deposited by
of will be deposited by
23. ⇒ (AIEEE 2003)
When during electrolysis of a solution of AgNO3, 9650 coulombs of charge pass through the electroplating bath, the mass of silver deposited on the cathode will be :
A. 10.8 g
B. 21.6 g
C. 108 g
D. 1.08 g
Correct Answer is Option (A)
When coulomb of electricity is passed through the
electroplating bath the amount of Ag deposited
when coulomb of electricity is passed deposited
Ag.
24. ⇒ (AIEEE 2002)
When the sample of copper with zinc impurity is to be purified by electrolysis, the appropriate electrodes are :
A. cathode = pure zinc, anode = pure copper
B. cathode = impure sample, anode = pure copper
C. cathode = impure zinc, anode = impure sample
D. cathode = pure copper, anode = impure sample
Correct Answer is Option (D)
Pure metal always deposits at cathode.