Correct answer is (D)
From (1) and (2)
6. (JEE Main 2023 (Online) 1st February Morning Shift )
A sample of gas at temperature is adiabatically expanded to double its volume. The work done by the gas in the process is :
(A)
(B)
(C)
(D)
Correct answer is (D)
From (1) and (2)
7. (JEE Main 2023 (Online) 31st January Evening Shift )
A hypothetical gas expands adiabatically such that its volume changes from 08 litres to 27 litres. If the ratio of final pressure of the gas to initial pressure of the gas is . Then the ratio of will be.
(A)
(B)
(C)
(D)
Correct answer is (B)
Let
be the ratio of
Then for adiabatic process
8. (JEE Main 2023 (Online) 30th January Morning Shift )
Heat is given to an ideal gas in an isothermal process.
A. Internal energy of the gas will decrease.
B. Internal energy of the gas will increase.
C. Internal energy of the gas will not change.
D. The gas will do positive work.
E. The gas will do negative work.
Choose the correct answer from the options given below :
(A) B and D only
(B) C and E only
(C) A and E only
(D) C and D only
Correct answer is (D)
Isothermal process
No change in internal energy
(1 law)
9. (JEE Main 2023 (Online) 25th January Evening Shift )
Match List I with List II
| List I | List II | ||
|---|---|---|---|
| A. | Isothermal Process | I. | Work done by the gas decreases internal energy |
| B. | Adiabatic Process | II. | No change in internal energy |
| C. | Isochoric Process | III. | The heat absorbed goes partly to increase internal energy and partly to do work |
| D. | Isobaric Process | IV. | No work is done on or by the gas |
Choose the correct answer from the options given below :
(A) A-I, B-II, C-IV, D-III
(B) A-II, B-I, C-III, D-IV
(C) A-II, B-I, C-IV, D-III
(D) A-I, B-II, C-III, D-IV
Correct answer is (C)
For isothermal process
is constant
So
Adiabatic process
Work done by gas is positive
So
is negative
For Isochoric process
For Isobaric process
Heat absorbed goes partly to increase internal energy and partly to do work.
10. (JEE Main 2022 (Online) 29th July Evening Shift )
A thermodynamic system is taken from an original state D to an intermediate state E by the linear process shown in the figure. Its volume is then reduced to the original volume from E to F by an isobaric process. The total work done by the gas from D to E to F will be
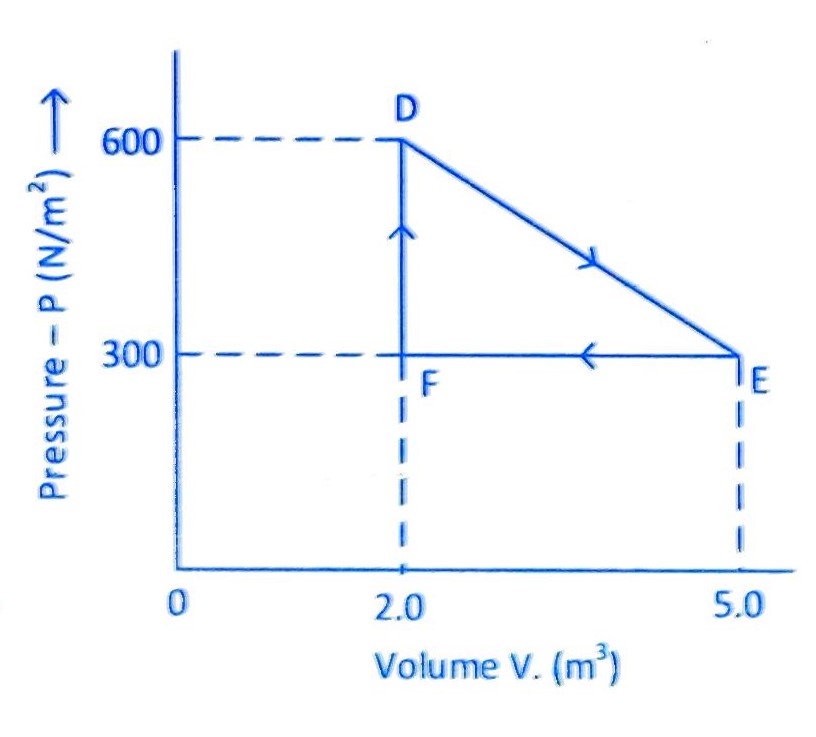
(A) 450 J
(B) 450 J
(C) 900 J
(D) 1350 J
Correct answer is (B)