Correct answer is (C)
constant
where
11. (JEE Main 2022 (Online) 26th July Morning Shift )
A monoatomic gas at pressure and volume is suddenly compressed to one eighth of its original volume. The final pressure at constant entropy will be :
(A) P
(B) 8P
(C) 32P
(D) 64P
Correct answer is (C)
constant
where
12. (JEE Main 2022 (Online) 25th July Morning Shift )
A certain amount of gas of volume at temperature and pressure expands isothermally until its volume gets doubled. Later it expands adiabatically until its volume gets redoubled. The final pressure of the gas will be (Use :
(A)
(B)
(C)
(D)
Correct answer is (B)
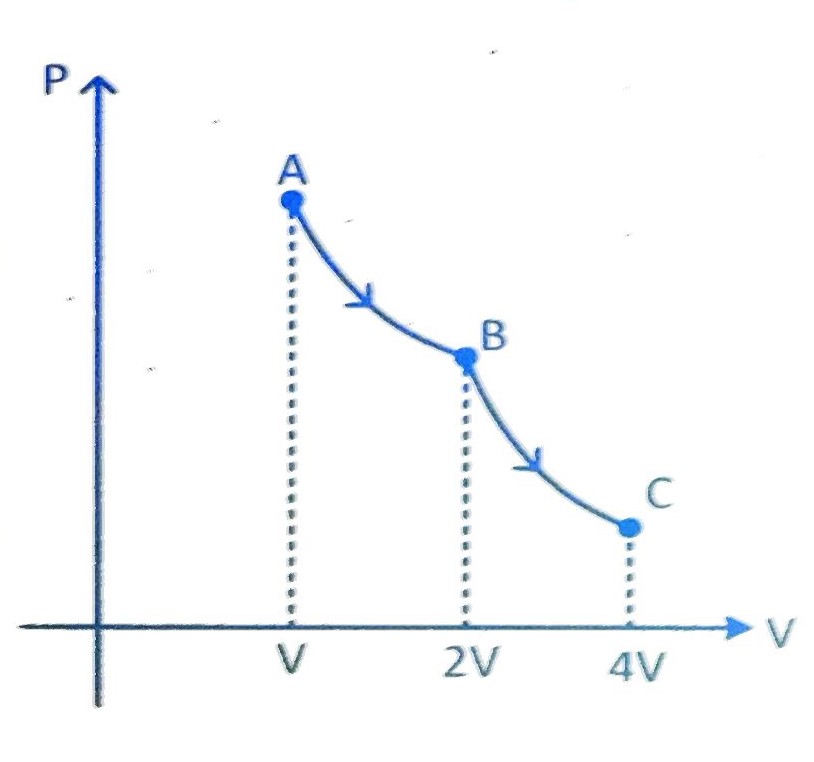
Let AB is isothermal process and BC is adiabatic process then for AB process
PAVA = PBVB
PB = 107 Nm2
For process BC
PBV = PC V
PC = 3.536 x 106 Pa
13. (JEE Main 2022 (Online) 30th June Morning Shift )
A sample of monoatomic gas is taken at initial pressure of 75 kPa. The volume of the gas is then compressed from 1200 cm3 to 150 cm3 adiabatically. In this process, the value of workdone on the gas will be :
(A) 79 J
(B) 405 J
(C) 4050 J
(D) 9590 J
Correct answer is (B)
For monoatomic gas degree of freedom f = 3 and =
Here for gas,
Initial pressure (P1) = 75 kPa
Initial volume (V1) = 1200 cm3
Final volume (V2) = 150 cm3
Final pressure (P2) = ?
For adiabatic process,
kPa
= 2400 kPa
Work done in adiabatic process,
kJ
14. (JEE Main 2022 (Online) 29th June Evening Shift )
Starting with the same initial conditions, an ideal gas expands from volume V1 to V2 in three different ways. The work done by the gas is W1 if the process is purely isothermal, W2, if the process is purely adiabatic and W3 if the process is purely isobaric. Then, choose the correct option
(A) W1 < W2 < W3
(B) W2 < W3 < W1
(C) W3 < W1 < W2
(D) W2 < W1 < W3
Correct answer is (D)
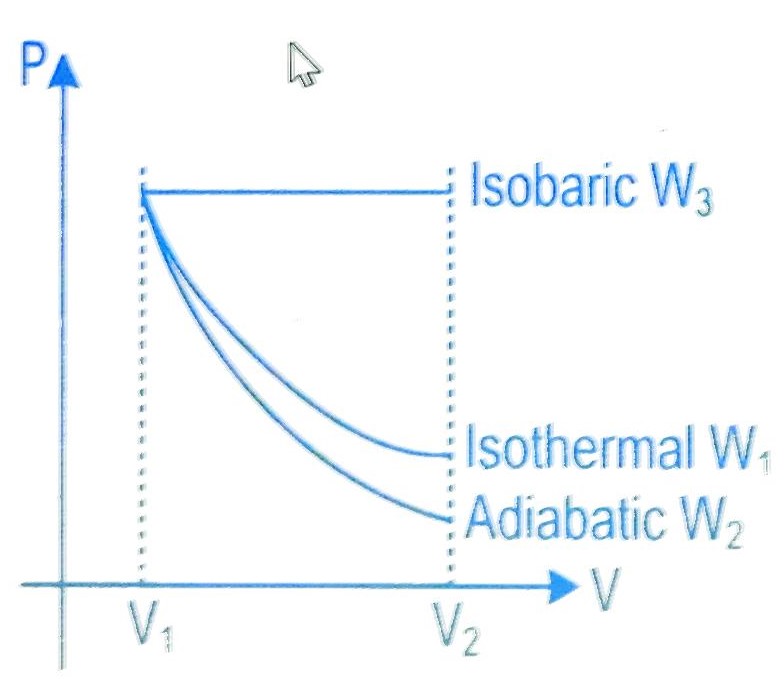
Comparing the area under the PV graph
A3 > A1 > A2
W3 > W1 > W2
15. (JEE Main 2022 (Online) 28th June Morning Shift )
Given below are two statements :
Statement I : When amount of an ideal gas undergoes adiabatic change from state (P1, V1, T1) to state (P2, V2, T2), then work done is , where and R = universal gas constant.
Statement II : In the above case, when work is done on the gas, the temperature of the gas would rise.
Choose the correct answer from the options given below :
(A) Both Statement I and Statement II are true.
(B) Both Statement I and Statement II are false.
(C) Statement I is true but Statement II is false.
(D) Statement I is false but Statement II is true.
Correct answer is (A)
for a polytropic process for adiabatic process r =
Statement I is true.
In an adiabatic process
U = W
If work is done on the gas
W is negative
U is positive or temperature increases
Statement II is true