Correct answer is (D)
w =
nRT
H = (Cv +
nR)T
26. (JEE Main 2019 (Online) 10th April Morning Slot )
n moles of an ideal gas with constant volume heat capcity CV undergo an isobaric expansion by certain volume. The ratio of the work done in the process, to the heat supplied is :
(A)
(B)
(C)
(D)
Correct answer is (D)
w =
nRT
H = (Cv +
nR)T
27. (JEE Main 2019 (Online) 9th April Morning Slot )
Following figure shows two processes A and
B for a gas. If
QA and
QB are the amount of
heat absorbed by the system in two cases, and
UA and
UB are changes in internal energies,
respectively, then :
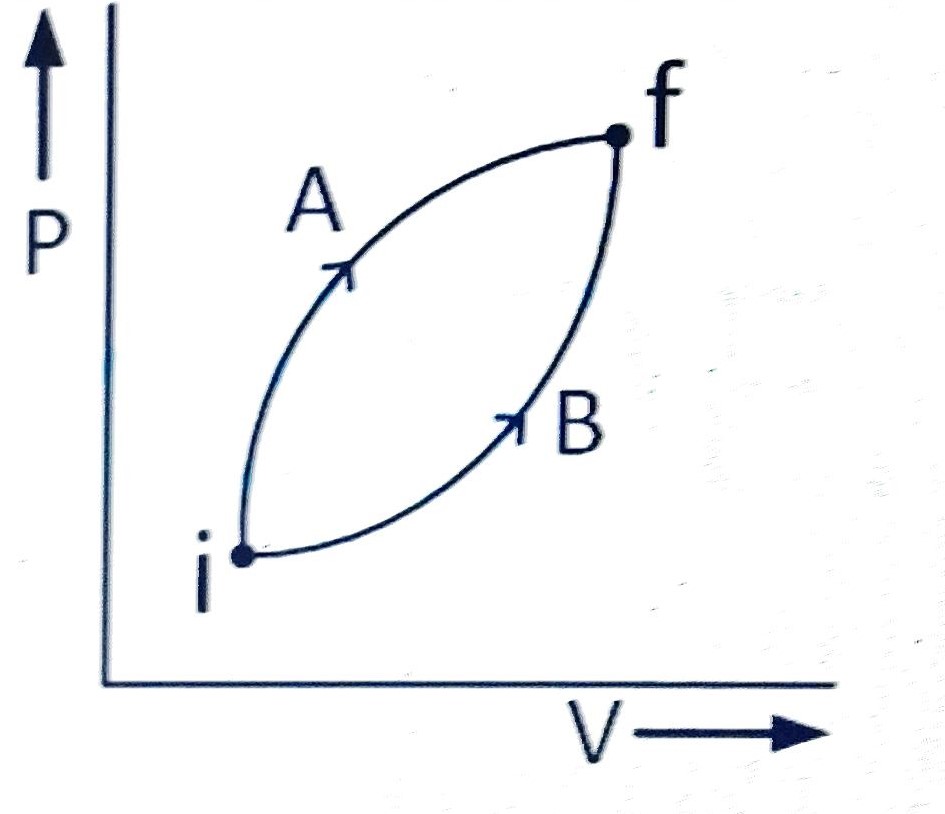
(A) QA > QB ; UA > UB
(B) QA < QB ; UA < UB
(C) QA > QB ; UA = UB
(D) QA = QB ; UA = UB
Correct answer is (C)
Initial and final states for both the processes are
same,
UA =
UB
Work done during process A is greater than in
process B. Because area is more
By First law of thermodynamics
Q =
U + W
QA >
QB
28. (JEE Main 2019 (Online) 12th January Morning Slot )
For the given cyclic process CAB as shown for a gas, the work done is :
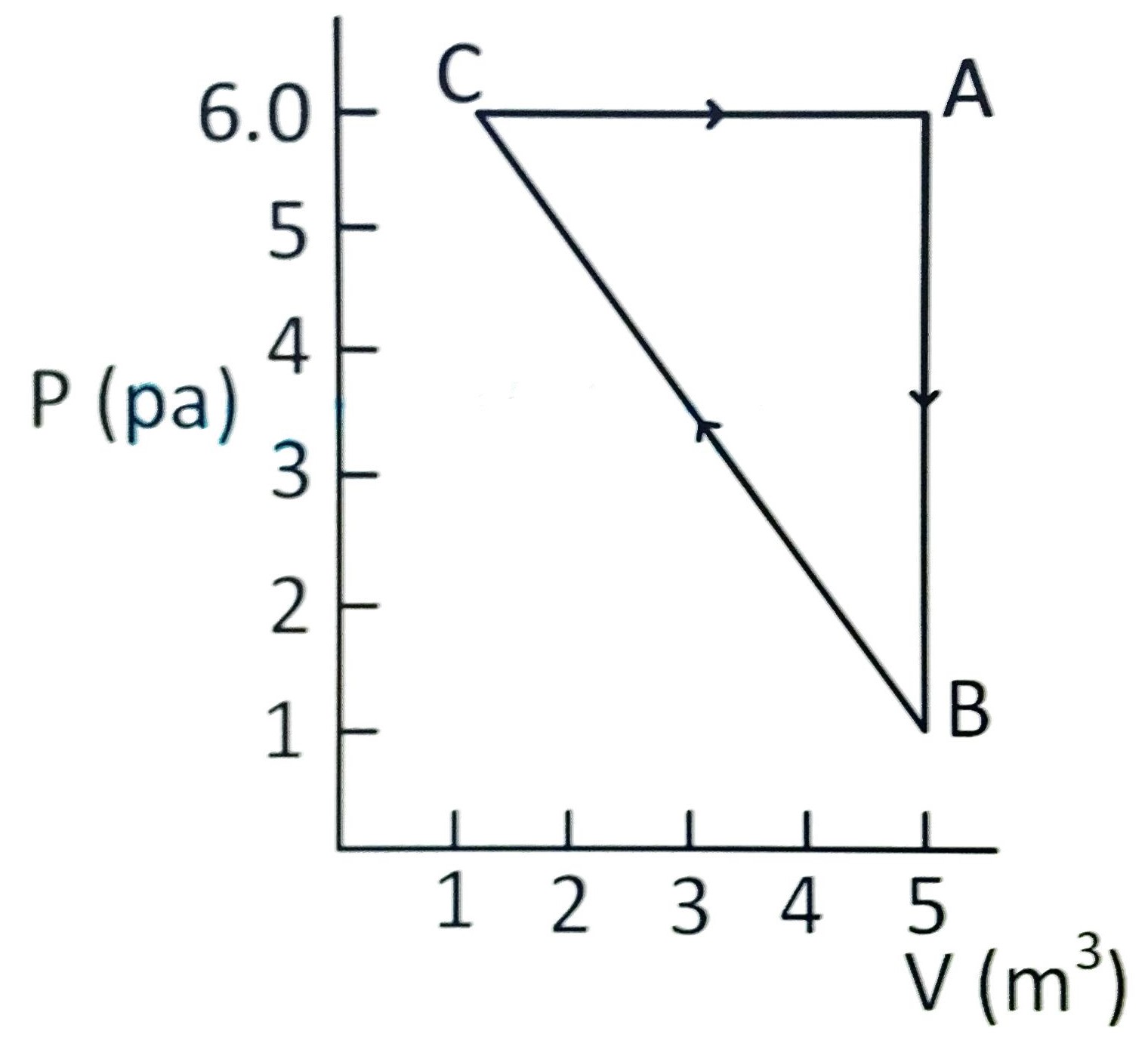
(A) 1 J
(B) 10 J
(C) 5 J
(D) 30 J
Correct answer is (B)
Since
PV indicator diagram is given, so work done by
gas
is area under the cyclic diagram.
W = Work done by gas =
4
5 J
= 10 J
29. (JEE Main 2019 (Online) 9th January Morning Slot )
A gas can be taken from A to B via two different processes ACB and ADB.
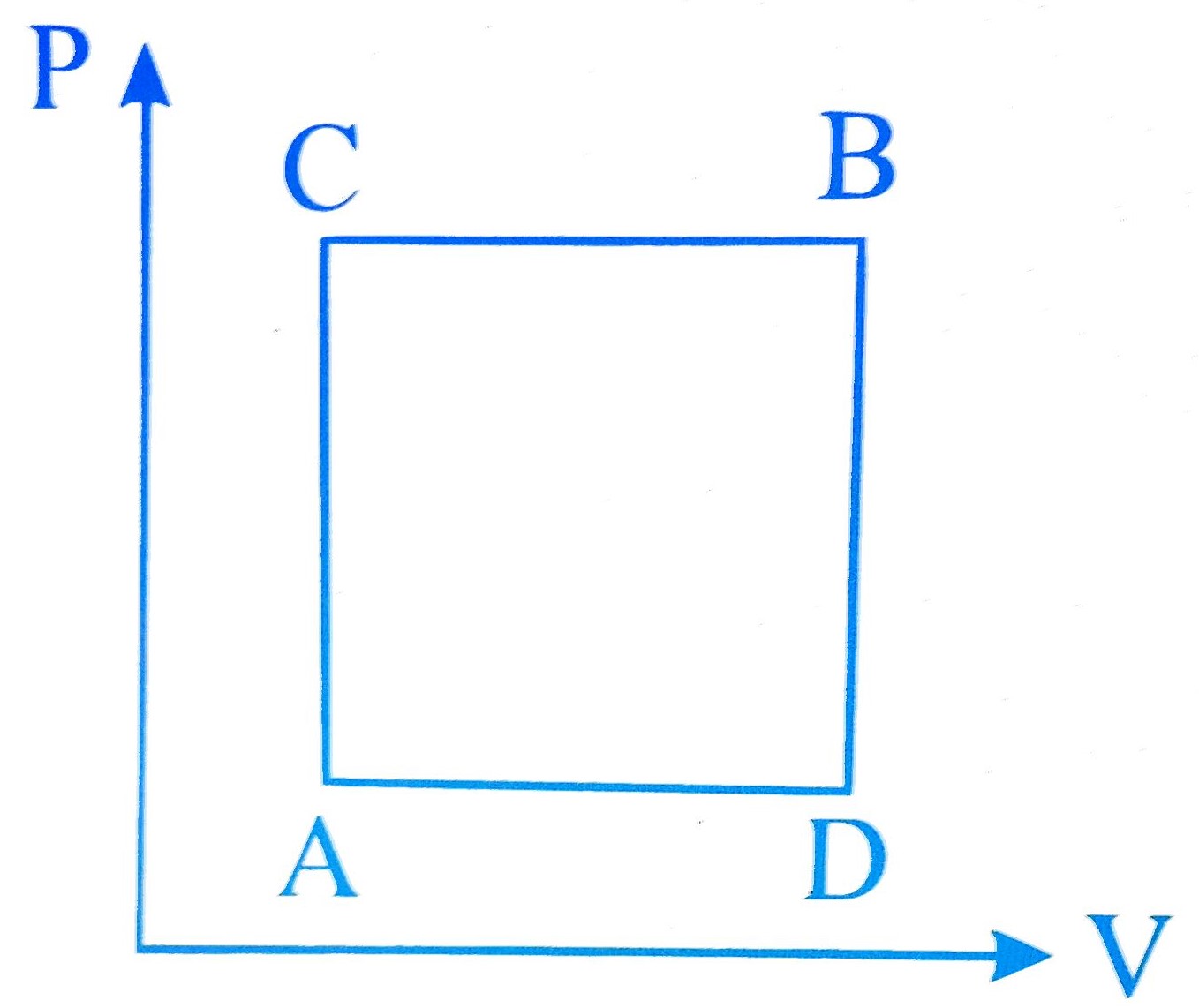
When path ACB is used 60 J of heat flows into the system and 30 J of work is done by the system.
If
path ADB is used work done by the system is 10 J. The heat Flow into the system in path ADB is :
(A) 40 J
(B) 80 J
(C) 100 J
(D) 20 J
Correct answer is (A)
Using law of thermodynamics in path ACB,
QACB =
UACB +
WACB
60 =
UACB + 30
UACB = 30 J
As value of
U is path independent,
UACB =
UADB = 30 J
In path ADB,
QADB =
UADB +
WADB
QADB = 30 + 10 = 40 J
30. (JEE Main 2016 (Online) 9th April Morning Slot )
200 g water is heated from 40oC to 60oC. Ignoring the slight expansion of water, the change in its internal energy is close to (Given specific heat of water = 4184 J/kg/K) :
(A) 8.4 kJ
(B) 4.2 kJ
(C) 16.7 kJ
(D) 167.4 kJ
Correct answer is (C)
According to the first law of thermodynamics,
Q =
u + w
For isochoric process Q =
U =
mst
T = (233
213) = 20 k
u = 0.2
4184
20 = 16.7 kJ