Correct Option is (C)
Chloroform (trichloromethane) when exposed to air and light forms a poisonous compound phosgene so it is stored in dark coloured air tight bottles.
1. ⇒ (MHT CET 2023 12th May Evening Shift )
Which of the following gases is formed during oxidation of trichloromethane?
A.
B.
C.
D.
Correct Option is (C)
Chloroform (trichloromethane) when exposed to air and light forms a poisonous compound phosgene so it is stored in dark coloured air tight bottles.
2. ⇒ (MHT CET 2023 12th May Evening Shift )
Which of the following compounds has difficulty in breaking of bond during nucleophilic substitution reaction?
A. o-Nitrochlorobenzene
B. p-Nitrochlorobenzene
C. m-Nitrochlorobenzene
D. 2,4,6-Trinitrochlorobenzene
Correct Option is (C)
Greater the number of electron withdrawing groups at o/p position, greater is the reactivity towards nucleophilic substitution reaction. Electron withdrawing group at meta position has practically no effect on reactivity. Hence, among the given, m-nitrochlorobenzene has difficulty in breaking of bond during nucleophilic substitution.
3. ⇒ (MHT CET 2023 12th May Morning Shift )
Which of the following compounds does NOT undergo Williamson's synthesis?
A.
B.
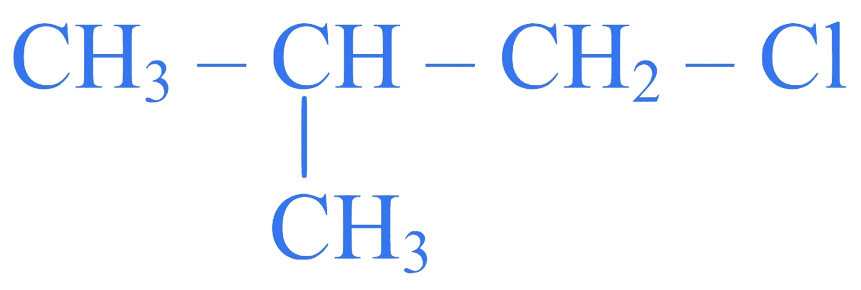
C.
D.
Correct Option is (C)
Aryl halides do not give Williamson's synthesis.
4. ⇒ (MHT CET 2023 12th May Morning Shift )
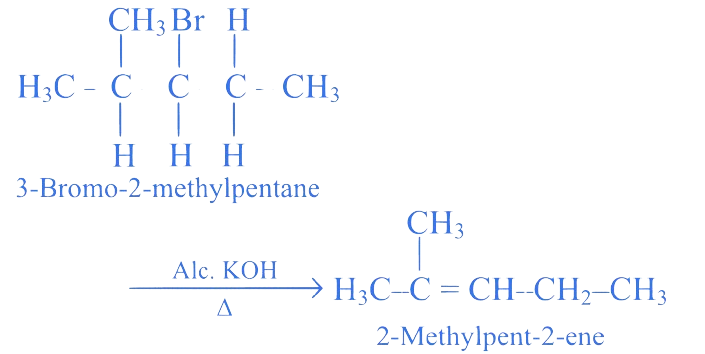
Identify major product in following reaction.
3-Bromo-2-methylpentane A
A. 2-Methylpentan-3-ol
B. 2-Methylpent-2-ene
C. 4-Methylpent-3-ene
D. 4-Methylpentan-3-ol
Correct Option is (B)
When alkyl halide having at least one -hydrogen is boiled with alcoholic solution of potassium hydroxide, it undergoes elimination of hydrogen atom from -carbon and halogen atom from -carbon resulting in the formation of an alkene. This reaction is known as dehydrohalogenation reaction or -elimination. The preferred product is that alkene which has greater number of alkyl groups attached to doubly bonded carbon atoms according to Saytzeff rule.
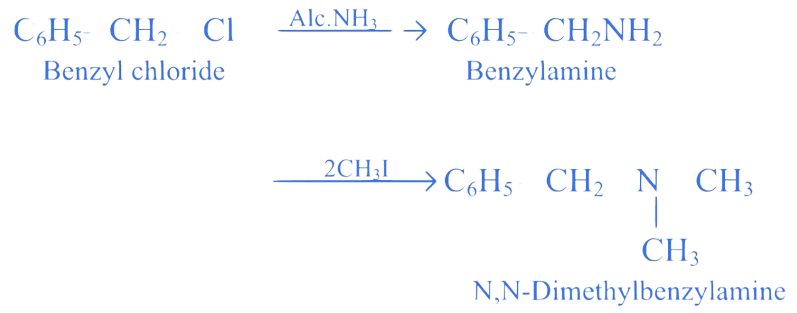
5. ⇒ (MHT CET 2023 11th May Evening Shift )
Identify the final product formed on ammonolysis of benzyl chloride followed by the reaction with two moles of .
A.
B.
C.
D.
Correct Option is (D)