Correct Option is (D)
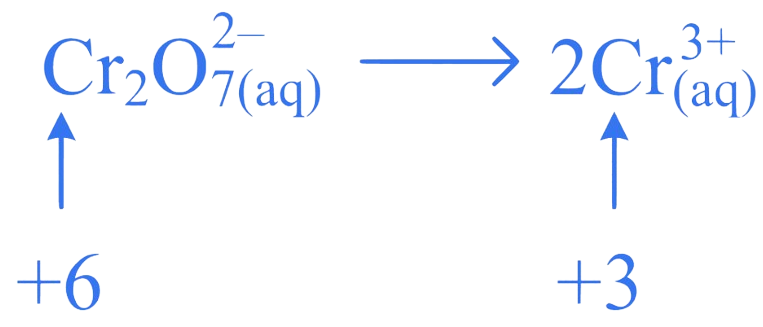
1. ⇒ (MHT CET 2023 12th May Evening Shift )
What is the change in oxidation number of in the following redox reaction?
A. +2 to +3
B. 2 to +3
C. +7 to +3
D. +6 to +3
Correct Option is (D)
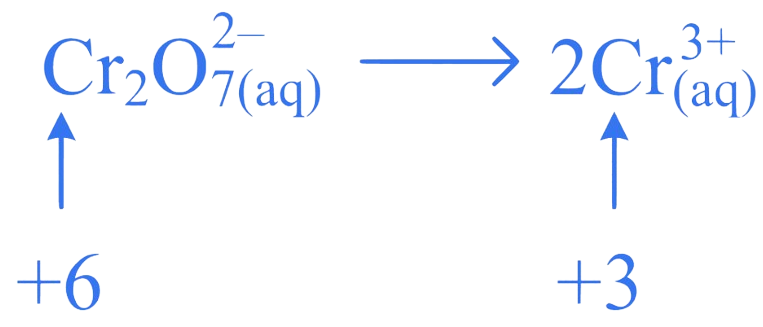
2. ⇒ (MHT CET 2023 11th May Evening Shift )
What is the oxidation number of sulfur in ?
A. +4
B. +6
C. +8
D. +5
Correct Option is (B)
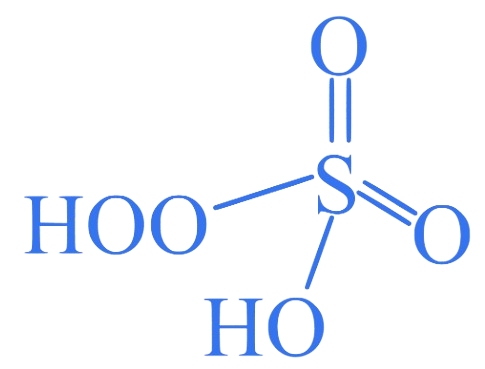
It has a peroxide linkage.
3. ⇒ (MHT CET 2023 10th May Evening Shift )
Identify the elements undergoing reduction and oxidation respectively in the following redox reaction.
A. As and
B. and
C. and
D. and
Correct Option is (B)
The oxidation number of decreases from +5 to 1 and that of As increases from +3 to +5.
Hence, undergoes reduction and undergoes oxidation.
4. ⇒ (MHT CET 2023 10th May Morning Shift )
What is the value of in order to balance following redox reaction?
A.
B.
C.
D.
Correct Option is (B)
The balanced equation is:
5. ⇒ (MHT CET 2023 9th May Morning Shift )
What is the oxidation state of carbon in and respectively?
A. and
B. and
C. and
D. and
Correct Option is (B)