Correct Option is (B)
Potassium dichromate Consider, oxidation state of is .
Oxidation state of
6. ⇒ (MHT CET 2021 21th September Morning Shift )
Oxidation state of Cr in potassium dichromate is
A. +7
B. +6
C. +1
D. +5
Correct Option is (B)
Potassium dichromate Consider, oxidation state of is .
Oxidation state of
7. ⇒ (MHT CET 2021 20th September Evening Shift )
What is the value of '' in order to balance the following redox reaction by ion electron method?
A. 3
B. 4
C. 1
D. 2
Correct Option is (D)
8. ⇒ (MHT CET 2021 20th September Morning Shift )
Identify reductant in following reaction.
A.
B.
C.
D.
Correct Option is (C)
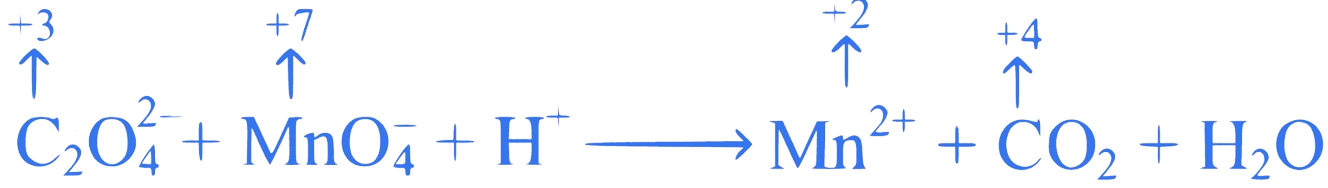
The oxidation number of C increases from to in above reaction.
acts as a reductant.