Correct Option is (B)
According to Charles's Law, the volume of an ideal gas is directly proportional to its absolute temperature when pressure is held constant. i.e.,
This is depicted by graph .
1. ⇒ (MHT CET 2023 13th May Morning Shift )
Which one of the following represents correctly the variation of volume (V) of an ideal gas with temperature under constant pressure conditions?
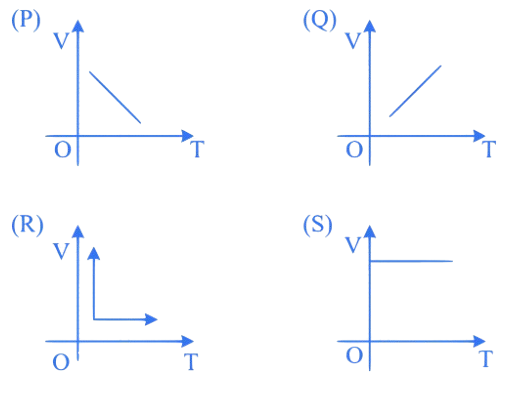
A. P
B. Q
C. R
D. S
Correct Option is (B)
According to Charles's Law, the volume of an ideal gas is directly proportional to its absolute temperature when pressure is held constant. i.e.,
This is depicted by graph .
2. ⇒ (MHT CET 2023 13th May Morning Shift )
For an ideal gas the density of the gas is when temperature and pressure of the gas are and respectively. When the temperature of the gas is , its pressure will be . The new density will be
A.
B.
C.
D.
Correct Option is (A)
Density
So,
The new density is:
3. ⇒ (MHT CET 2023 11th May Evening Shift )
According to Boyle's law, the product PV remains constant. The unit of is same as that of
A. energy
B. force
C. impulse
D. momentum
Correct Option is (A)
The units of PV can be calculated as follows:
The unit of pressure is
The unit of volume is
The unit of PV is
Unit
This is a unit of energy.
4. ⇒ (MHT CET 2023 10th May Evening Shift )
We have a jar filled with gas characterized by parameters and another jar B filled with gas having parameters , where symbols have their usual meaning. The ratio of number of molecules in jar A to those in jar B is
A.
B.
C.
D.
Correct Option is (D)
According to the gas equation,
For the first gas, we have,
..... (i)
For the second gas, we have,
From equations (i) and (ii)
5. ⇒ (MHT CET 2023 10th May Morning Shift )
The average force applied on the walls of a closed container depends on where is the temperature of an ideal gas. The value of '' is
A. 4
B. 3
C. 2
D. 1
Correct Option is (D)
From Ideal Gas Equation,
When V remains constant,
..... (ii)
Comparing (i) and (ii)