Correct Option is (A)
After isothermal expansion:
After adiabatic expansion:
6. ⇒ (MHT CET 2023 11th May Morning Shift )
A monoatomic gas at pressure '', having volume '' expands isothermally to a volume '' and then adiabatically to a volume ''. The final pressure of the gas is (Take )
A.
B.
C.
D.
Correct Option is (A)
After isothermal expansion:
After adiabatic expansion:
7. ⇒ (MHT CET 2023 11th May Morning Shift )
A diatomic gas is compressed adiabatically to volume where is its initial volume. The initial temperature of the gas is in Kelvin and the final temperature is ''. The value of '' is
A. 5
B. 4
C. 3
D. 2
Correct Option is (B)
For adiabatic process: Constant
Initially:
Final condition:
So,
8. ⇒ (MHT CET 2023 10th May Evening Shift )
Which of the following graphs between pressure (P) and volume (V) correctly shows isochoric changes?
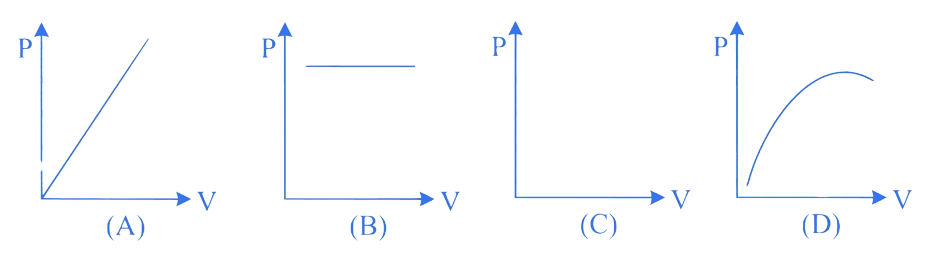
A. D
B. B
C. C
D. A
Correct Option is (C)
Isochoric processes are those in which the volume of the system remains constant. In a Pressure-Volume (P-V) graph, an isochoric process is represented by a vertical line because the volume does not change, while the pressure can vary.
Looking at the provided graph :
Therefore, the correct option that represents an isochoric process is :
Option C : C
9. ⇒ (MHT CET 2023 10th May Morning Shift )
A sample of gas at temperature is adiabatically expanded to double its volume. The work done by the gas in the process is gas constant
A.
B.
C.
D.
Correct Option is (D)
Using the formula for adiabatic expansion,
Work done
10. ⇒ (MHT CET 2023 9th May Evening Shift )
A gas at N.T.P. is suddenly compressed to onefourth of its original volume. If , then the final pressure is
A. 4 times
B. 1.5 times
C. 8 times
D. times
Correct Option is (C)
As the change is sudden, the process is adiabatic