Correct Option is (B)
De-Broglie wavelength where is momentum
For an electron velocity ,
hence
16. ⇒ (MHT CET 2021 21th September Morning Shift )
When an electron in hydrogen atom jumps from third excited state to the ground state, the de-Broglie wavelength associated with the electron becomes
A.
B.
C.
D.
Correct Option is (B)
De-Broglie wavelength where is momentum
For an electron velocity ,
hence
17. ⇒ (MHT CET 2021 21th September Morning Shift )
'' is the wavelength of series limit of Lyman series, '' is the wavelength of the first line line of Lyman series and '' is the series limit of the Balmer series. Then the relation between and is
A.
B.
C.
D.
Correct Option is (A)
Series limit of Lyman series is given by
Series limit of Balmer series is given by
First line of Lyman series is given by
18. ⇒ (MHT CET 2021 20th September Evening Shift )
The P.E. 'U' of a moving particle of mass 'm' varies with 'x'-axis as shown in figure. The deBroglie wavelength or the particle in the regions and are and respectively. II the total energy of the particle is '', then the ratio is
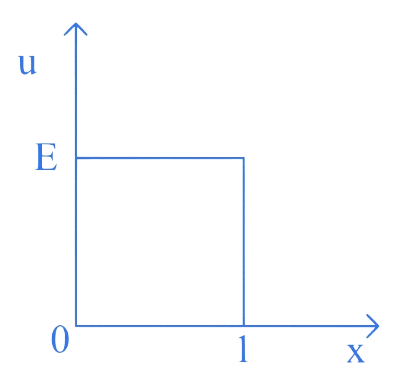
A.
B.
C.
D.
Correct Option is (C)
In the region , the potential energy of the particle is .
Total energy is .
Hence, kinetic energy,
Its momentum,
In the region is zero.
Hence, total energy is kinetic energy.
19. ⇒ (MHT CET 2021 20th September Evening Shift )
The gyromagnetic ratio of an electron in an hydrogen atom, according to Bohr model is
A. decreases with the quantum number 'n'.
B. independent of which orbit it is in.
C. negative
D. positive
Correct Option is (B)
Gyromagnetic ratio =
It is independent of the orbit of the electron.
20. ⇒ (MHT CET 2021 20th September Morning Shift )
The electron in hydrogen atom is initially in the third excited state. When it finally moves to ground state, the maximum number of spectral lines emitted are
A. 3
B. 4
C. 5
D. 6
Correct Option is (D)
Initially the electron is in the third excited state i.e. n = 4. Hence we can have transitions :
Hence, six transitions are possible.