correct answer is 28
# Explanation
Cell reaction is :
Now, .... (1)
....(2)
From (1) and (2), E2 =
0.28 V = 28
102 V
33. ⇒ (JEE Main 2021 (Online) 27th July Evening Shift )
For the cell
Cu(s) | Cu2+ (aq) (0.1 M) ||
Ag+(aq) (0.01 M) | Ag(s)
the cell potential E1 = 0.3095 V
For the
cell
Cu(s) | Cu2+ (aq) (0.01 M) || Ag+(aq) (0.001 M) | Ag(s)
the
cell
potential = ____________ 102 V. (Round off the nearest integer).
[Use :
= 0.059]
correct answer is 28
# Explanation
Cell reaction is :
Now, .... (1)
....(2)
From (1) and (2), E2 =
0.28 V = 28
102 V
34. ⇒ (JEE Main 2021 (Online) 25th July Morning Shift )
Consider the cell at 25C
Zn | Zn2+ (aq), (1M) || Fe3+
(aq), Fe2+ (aq) | Pt(s)
The fraction of total iron present as Fe3+ ion at
the
cell
potential of 1.500 V is x 102. The value of x is ______________. (Nearest
integer)
(Given : , )
correct answer is 24
# Explanation
V
= 0.2402
= 24 10-2
35. ⇒ (JEE Main 2021 (Online) 22th July Evening Shift )
Assume a cell with the following reaction
V
Ecell for the above reaction is
______________ V. (Nearest integer)
[Given : log 2.5 = 0.3979, T = 298 K]
correct answer is 3
# Explanation
36. ⇒ (JEE Main 2021 (Online) 18th March Morning Shift )
For the reaction
2Fe3+(aq) + 2I(aq) 2Fe2+(aq) + I2(s)
the
magnitude of the standard molar Gibbs free energy change, rG = ___________ kJ (Round off to the Nearest
Integer).
correct answer is 46
# Explanation
37. ⇒ (JEE Main 2021 (Online) 26th February Evening Shift )
Emf of the following cell at 298K in V is x 102.
Zn|Zn2+(0.1 M)||Ag+
(0.01 M)|Ag
The value of x is _________. (Rounded off to the nearest integer)
[Given :
]
correct answer is 147
# Explanation
Zn | Zn2+(0.1 M) || Ag+ (0.01 M) | Ag
Zn(s) + 2Ag+ 2Ag(s) + Zn+2
38. ⇒ (JEE Main 2021 (Online) 25th February Evening Shift )
Copper reduces NO into NO and NO2 depending upon the concentration
of HNO3 in solution. (Assuming fixed [Cu2+] and PNO =
PNO2), the HNO3 concentration at which the thermodynamic
tendency
for reduction of NO into NO and NO2 by copper is same is
10x M. The value of 2x is _______. (Rounded off to the nearest integer)
[Given, V, V, V and at 298 K, (2.303) = 0.059]
correct answer is 1
# Explanation
Cell-I
Cell-II
Now,
[ ]
Now,
So,
39. ⇒ (JEE Main 2021 (Online) 24th February Evening Shift )
The magnitude of the change in oxidising power of the couple is x 104 V, if the H+ concentration is decreased from 1M to 104 M at 25C. (Assume concentration of and to be same on change in H+ concentration). The value of x is ___________.
correct answer is 3776
# Explanation
Reaction,
n = 5
Applying Nernst equation,
or
(I) Given, [H+] = 1
M
(II) Now, [H+] = 104 M
x = 3776
40. ⇒ (JEE Main 2021 (Online) 24th February Morning Shift )
The electrode potential of M2+/M of 3d-series elements shows positive value for :
(A) Zn
(B) Fe
(C) Cu
(D) Co
Explanation
Correct answer is C
In the electrode potential series, only copper have positive value
for
electrode potential because copper has lower tendency than hydrogen to form ions. So, if
standard
hydrogen electrode (ECell = 0) is connected to copper half-cell, the copper with
be
relatively less negative or less number of electrons.
= + 0.34 V; = 0.41 V
= 0.28 V; = 0.76 V
Electrode potential of Cu show positive value.
41. ⇒ (JEE Main 2020 (Online) 6th September Evening Slot )
For the given cell :
Cu(s) | Cu2+(C1M) || Cu2+(C2M) | Cu(s)
change in Gibbs energy (G) is negative, if :
(A) C2 = C1
(B) C2 =
(C) C1 = 2C2
(D) C1 = C2
Explanation
Correct answer is A
Given G < 0
-nFEcell < 0
Ecell > 0
We know, Ecell = -
= 0 -
- > 0
< 0
C1 < C2
By checking option, we can see
C2 = C1 satisfy the condition C1
< C2.
42. ⇒ (JEE Main 2020 (Online) 4th September Morning Slot )
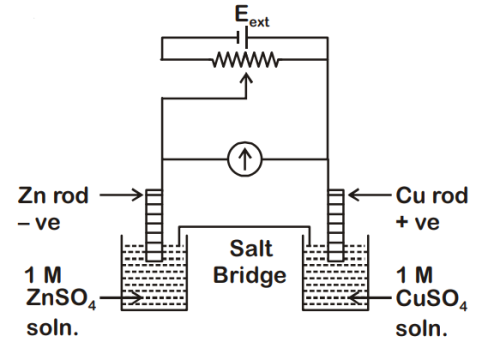
= +0.34 V
= -0.76 V
Identify the incorrect statement from the option
below for the above cell :
(A) If Eext < 1.1 V, Zn dissolves at anode and Cu deposits at cathode
(B) If Eext = 1.1 V, no flow of e– or current occurs
(C) If Eext > 1.1 V, e– flows from Cu to Zn
(D) If Eext > 1.1 V, Zn dissolves at Zn electrode and Cu deposits at Cu electrode
Explanation
Correct answer is D
= -
= 0.34 – (–0.76)
= 1.10 V
If Eext < 1.1 V then Zn dissolves at anode and
copper deposits at Cathode.
If Eext > 1.1V then Zn deposited at zinc
electrodes and Cu deposits at Cu electrode.