correct answer is 7
# Explanation
22. ⇒ (JEE Main 2022 (Online) 29th June Evening Shift )
The cell potential for the given cell at 298 K
Pt| H2 (g, 1 bar) | H+ (aq) || Cu2+ (aq) | Cu(s)
is 0.31 V. The pH of the acidic solution is found to be 3, whereas the concentration of Cu2+ is 10x M. The value of x is ___________.
(Given : = 0.34 V and = 0.06 V)
correct answer is 7
# Explanation
23. ⇒ (JEE Main 2022 (Online) 28th June Evening Shift )
For the given reactions
Sn2+ + 2e Sn
Sn4+ + 4e Sn
the electrode potentials are ; V and V. The magnitude of standard electrode potential for i.e. is _____________ 102 V. (Nearest integer)
correct answer is 16
# Explanation
24. ⇒ (JEE Main 2022 (Online) 27th June Evening Shift )
For the reaction taking place in the cell :
Pt (s)| H2 (g)|H+(aq) || Ag+(aq) |Ag (s)
E = + 0.5332 V.
The value of fG is ______________ kJ mol1. (in nearest integer)
correct answer is 51 or 103
# Explanation
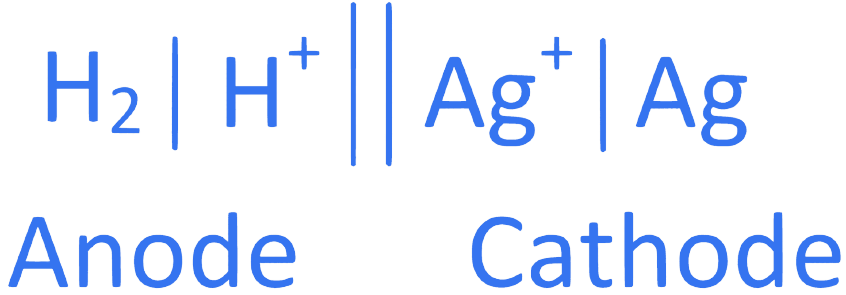
# At anode, oxidation occur
H2 2H+ + 2e ....... (1)
# At cathode, reduction occur
2Ag+ + 2e 2Ag ...... (2)
Adding equation (1) and (2), we get n = 2, where n = cancelled out electron
Now,
kJ/mol
kJ/mol
25. ⇒ (JEE Main 2022 (Online) 26th June Morning Shift )
The of different types of half cells are as follows:
| A | B | C | D |
|---|---|---|---|
(Where E is the electromotive force)
Which of the above half cells would be preferred to be used as reference electrode?
(A) A
(B) B
(C) C
(D) D
Explanation
Correct answer is C
A cell with less variation in EMF with temperature is preferred as a reference electrode because it can be used for a wider range of temperatures without much derivation from standard value so a cell with less is preferred.
26. ⇒ (JEE Main 2022 (Online) 26th June Evening Shift )
Cu(s) + Sn2+ (0.001M) Cu2+ (0.01M) + Sn(s)
The Gibbs free energy change for the above reaction at 298 K is x 101 kJ mol1. The value of x is __________. [nearest integer]
[Given : ; ; F = 96500 C mol1]
correct answer is 983
# Explanation
27. ⇒ (JEE Main 2022 (Online) 25th June Morning Shift )
In a cell, the following reactions take place
The standard electrode potential for the spontaneous reaction in the cell is x 102 V 298 K. The value of x is ____________. (Nearest Integer)
correct answer is 23
# Explanation
28. ⇒ (JEE Main 2022 (Online) 25th June Evening Shift )
The correct order of reduction potentials of the following pairs is
A. Cl2/Cl
B. I2/I
C. Ag+/Ag
D. Na+/Na
E. Li+/Li
Choose the correct answer from the options given below.
(A) A > C > B > D > E
(B) A > B > C > D > E
(C) A > C > B > E > D
(D) A > B > C > E > D
Explanation
Correct answer is A
29. ⇒ (JEE Main 2022 (Online) 24th June Morning Shift )
The cell potential for the following cell
Pt |H2(g)|H+ (aq)|| Cu2+ (0.01 M)|Cu(s)
is 0.576 V at 298 K. The pH of the solution is __________. (Nearest integer)
(Given : V and V)
correct answer is 5
# Explanation
30. ⇒ (JEE Main 2021 (Online) 31st August Morning Shift )
Consider the following cell reaction :
The value of is 4.315 V at 25C. If H = 825.2 kJ mol1, the standard entropy change S in J K1 is ___________. (Nearest integer) [Given :
Faraday constant = 96487 C mol1]
correct answer is 25
# Explanation
S
JK1 mol1
Nearest integer answer is 25.
31. ⇒ (JEE Main 2021 (Online) 26th August Evening Shift )
For the galvanic cell,
Zn(s) + Cu2+ (0.02 M)
Zn2+ (0.04 M) + Cu(s),
Ecell =
______________ 102 V. (Nearest integer)
[Use : = 0.34 V, = + 0.76 V, ]
correct answer is 109
# Explanation
Galvanic cell :
Nernst equation =
= 1.0911 = 109.11 102
= 109
32. ⇒ (JEE Main 2021 (Online) 26th August Morning Shift )
These are physical properties of an element
(A) Sublimation
enthalpy
(B) Ionisation enthalpy
(C) Hydration enthalpy
(D) Electron gain
enthalpy
The total number of above properties that affect the reduction potential is ____________
(Integer answer)
correct answer is 3
# Explanation
Sublimation enthalpy, Ionisation enthalpy and hydration enthalpy affect the reduction potential.