Correct answer is (D)
1. ⇒ (NEET 2023 Manipur )
A container of volume contains 0.2 mole of hydrogen gas and 0.3 mole of argon gas. The pressure of the system at temperature () will be :-
(A)
(B)
(C)
(D)
Correct answer is (D)
2. ⇒ (NEET 2022 Phase 1 )
The volume occupied by the molecules contained in 4.5 kg water at STP, if the intermolecular forces vanish away is
(A) 5.6 106 m3
(B) 5.6 103 m3
(C) 5.6 103 m3
(D) 5.6 m3
Correct answer is (D)
From ideal gas equation
At
N/m2
m3
3. ⇒ (NEET 2020 Phase 1 )
A cylinder contains hydrogen gas at pressure 249 kPa and
temperature 27C
Its density is : (R = 8.3 J mol-1
K-1)
(A) 0.2 kg/m3
(B) 0.1 kg/m3
(C) 0.02 kg/m3
(D) 0.5 kg/m3
Correct answer is (A)
From the ideal gas equation, PV = nRT also
Volume (V) =
So, PM = RT
P = 249 103 N/m2
M = 2
10-3 kg
T = 333
K
=
= 0.2 kg/m3
4. ⇒ (NEET 2016 Phase 2 )
A given sample of an ideal gas occupies a volume V at a pressure P and absolute temperature T. The mass of each molecule of the gas is m. Which of the following gives the density of the gas ?
(A) P/(kT)
(B) Pm/(kT)
(C) P/(KTV)
(D) mkT
Correct answer is (B)
As PV = nRT
...(i)
Density, =
[From eqn. (i)]
5. ⇒ (AIPMT 2015 )
Two vessels separately contain two ideal gases A and B at the same temperature, the pressure of A being twice that of B. Under such conditions, the density of A is found to be 1.5 times the density of B. The ratio of molecular weight of A and B is
(A) 2
(B)
(C)
(D)
Correct answer is (D)
From PV = nRT
and
From question,
6. ⇒ (NEET 2013 )
In the given (V T) diagram, what is the relation between pressure
P1 and P2?
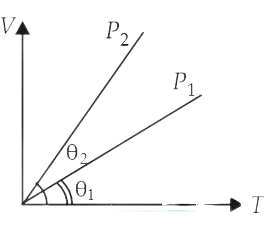
(A) P2 < P1
(B) Carnot be predicted
(C) P2 = P1
(D) P2 > P1
Correct answer is (A)
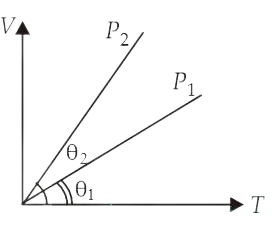
According to ideal gas equation PV = nRT
For an isobaric process, P = constant and V T
Therefore, V – T graph is a straight
line passing through origin. Slope of this line is inversely proportional to P.
In the given
figure,
(Slope)2 > (Slope)1 P2 < P1
7. ⇒ (AIPMT 2008 )
At 10oC the value of the density of a fixed mass of an ideal gas divided by it pressure is x. At 110oC this ratio is
(A)
(B)
(C)
(D)
Correct answer is (B)
PV = nRT
8. ⇒ (AIPMT 2004 )
The equation of state for 5 g of oxygen at a pressure P and
temperature T, when occupying a volume V, will be
(where R is the gas constant)
(A) PV = (5/32)RT
(B) PV = 5RT
(C) PV = (5/2)RT
(D) PV = (5/16) RT
Correct answer is (A)
As PV = nRT