Correct answer is (D)
(A)
(B)
(C)
(D)
1. ⇒ (NEET 2021 )
Match Column - I and Column - II and choose the correct match from
the given choices.
| Column - I | Column - II | ||
|---|---|---|---|
| (A) | Root mean square speed of gas molecules | (P) | |
| (B) | Pressure exerted by ideal gas | (Q) | |
| (C) | Average kinetic energy of a molecule | (R) | |
| (D) | Total internal energy of 1 mole of a diatomic gas | (S) |
(A) (A) - (R), (B) - (Q), (C) - (P), (D) - (S)
(B) (A) - (R), (B) - (P), (C) - (S), (D) - (Q)
(C) (A) - (Q), (B) - (R), (C) - (S), (D) - (P)
(D) (A) - (Q), (B) - (P), (C) - (S), (D) - (R)
Correct answer is (D)
(A)
(B)
(C)
(D)
2. ⇒ (NEET 2020 Phase 1 )
The average thermal energy for a mono-atomic gas is : (kB is Boltzmann constant and T absolute temperature)
(A)
(B)
(C)
(D)
Correct answer is (A)
The degree of freedom for monoatomic gas is 3. So, average
thermal energy per molecule,
K.E.avg =
3. ⇒ (NEET 2019 )
Increase in tempertaure of a gas filled in a container would lead to :
(A) increase in its kinetic energy
(B) decrease in intermolecular distance
(C) decrease in its pressure
(D) increase in its mass
Correct answer is (A)
Since, the increase of temperature will increase the kinetic
energy for the given gas.
For the given ideal gas,
U =
4. ⇒ (NEET 2017 )
A gas mixture consists of 2 moles of O2 and 4 moles of Ar at temperature T. Neglecting all vibrational modes, the total internal energy of the system is
(A) 15 RT
(B) 9 RT
(C) 11 RT
(D) 4 RT
Correct answer is (C)
Internal energy of the system is given by
Degree of freedom
Fdiatomic = 5
fmonoatomic = 3
and, number of moles n(O2) = 2
n(Ar) = 4
5. ⇒ (AIPMT 2015 Cancelled Paper )
One mole of an ideal diatomic gas undergoes a transition from A to
B along a path AB as shown in the figure.
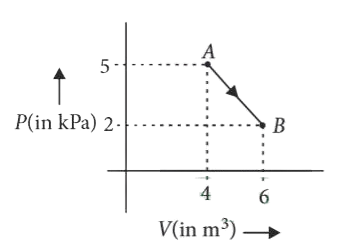
The change in internal energy of the gas during the transition is
(A) 20 J
(B) 12 kJ
(C) 20 kJ
(D) 20 kJ
Correct answer is (D)
Change in internal energy from A B
(As gas is diatomic f = 5)