Correct answer is (D)
is isobaric process
1. ⇒ (NEET 2023 Manipur )
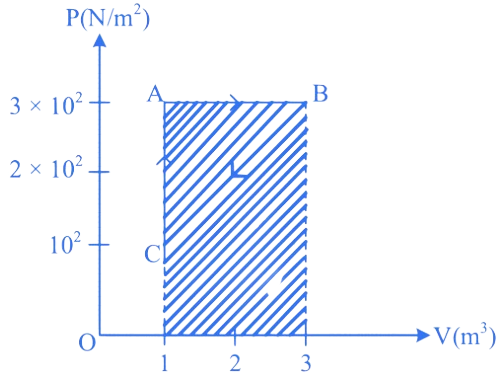
For the given cycle, the work done during isobaric process is:
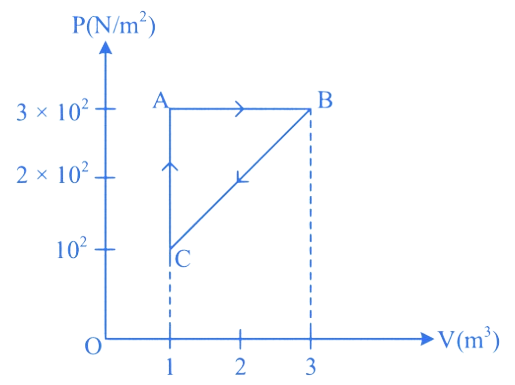
(A) 200 J
(B) Zero
(C) 400 J
(D) 600 J
Correct answer is (D)

is isobaric process
2. ⇒ (NEET 2022 Phase 2 )
An ideal gas follows a process described by the equation from the initial to final thermodynamic states, where C is a constant. Then
(A) If then
(B) If then
(C) If then
(D) If then
Correct answer is (D)
We know,
Given, constant
If
constant
constant
i.e., if
Also, constant
constant
If then
3. ⇒ (NEET 2022 Phase 1 )
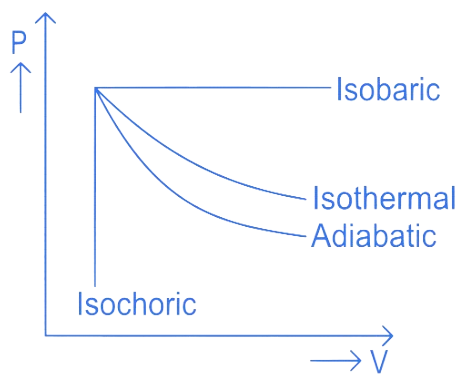
An ideal gas undergoes four different processes from the same initial state as shown in the figure below. Those processes are adiabatic, isothermal, isobaric and isochoric. The curve which represents the adiabatic process among 1, 2, 3 and 4 is
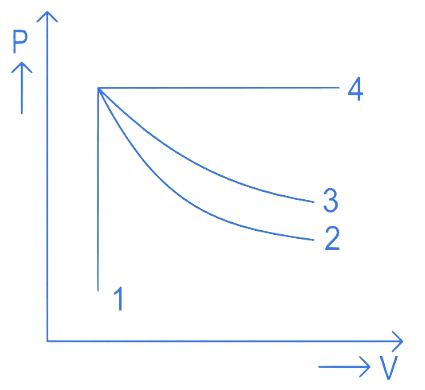
(A) 1
(B) 2
(C) 3
(D) 4
Correct answer is (B)
4. ⇒ (NEET 2020 Phase 1 )
Two cylinders A and B of equal capacity are connected to each other via a stop cock. A contains an ideal gas at standard temperature and pressure. B is completely evacuated. The entire systems is thermally insulated. The stop cock is suddenly opened. The Process is :
(A) adiabatic
(B) isochoric
(C) isobaric
(D) isothermal
Correct answer is (A)
The system is insulated thermally. So no heat exchange takes place. Therefore the process is adiabatic.
5. ⇒ (NEET 2019 )
In which of the following processes, heat is neither absorbed nor released by a system?
(A) adiabatic
(B) isobaric
(C) isochoric
(D) isothermal
Correct answer is (A)
From the characteristic property of adiabatic process, there is no exchange in heat.
6. ⇒ (NEET 2018 )
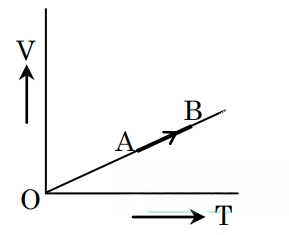
The volume (V) of a monatomic
gas varies with its temperature
(T), as shown in the graph. The
ratio of work done by the gas,
to the heat absorbed by it, when
it undergoes a change from state
A to state B, is
(A)
(B)
(C)
(D)
Correct answer is (A)
Gas is monatomic, so Cp =
Given process is isobaric.
dQ = nCpdT
or dQ = ndT
Also, dW = PdV = nRdT ( PV = nRT)
Required ratio = = =
7. ⇒ (NEET 2017 )
Thermodynamic processes are indicated in the following diagram.

Match the following
| Column-1 | Column-2 | |||
|---|---|---|---|---|
| P. | Process I | A. | Adiabatic | |
| Q. | Process II | B. | Isobaric | |
| R. | Process III | C. | Isochoric | |
| S. | Process IV | D. | Isothermal | |
(A) P C, Q A, R D, S B
(B) P C, Q D, R B, S A
(C) P D, Q B, R A, S C
(D) P A, Q C, R D, S B
Correct answer is (A)
Process I volume is constant hence, it is isochoric
In
process IV, pressure is constant hence, it is isobaric.
8. ⇒ (NEET 2016 Phase 2 )
One mole of an ideal monatomic gas undergoes a process described by the equation PV3 = constant. The heat capacity of the gas during this process is
(A) R
(B) R
(C) 2R
(D) R
Correct answer is (D)
Process described by the equation,
PV3 = constant
For a polytropic process, PV = constant
9. ⇒ (NEET 2016 Phase 1 )
A gas is compressed isothermally to half its initial volume. The same gas is compressed separately through an adiabatic process until its volume is again reduced to half. Then
(A) Compressing the gas isothermally or adiabatically will require the same amount of work.
(B) Which of the case (whether compression through isothermal or through adiabatic process) requires more work will depend upon the atomicity of the gas.
(C) Compressing the gas isothermally will require more work to be done.
(D) Compressing the gas through adiabatic process will require more work to be done.
Correct answer is (D)
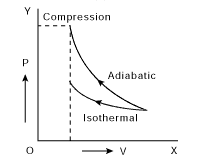
V1 = V, V2 = V/2
On P-V diagram, Area under adiabatic curve > Area under isothermal curve.
So
compressing the gas through adiabatic process will require more work to be done.
10. ⇒ (AIPMT 2015 )
An ideal gas is compressed to half its initial volume by means of several processes. Which of the process results in the maximum work done on the gas ?
(A) Isochoric
(B) Isothermal
(C) Adiabatic
(D) Isobaric
Correct answer is (C)
The P-V diagram of an ideal gas compressed from its initial
volume to by several processes is shown in the
figure.
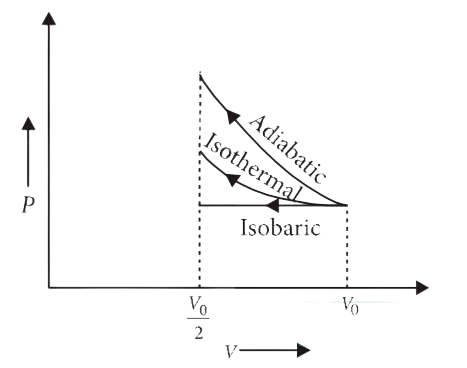
Work done on the gas = Area under P–V curve
As area under the P–V curve is maximum for
adiabatic process, so work done on the gas is maximum for adiabatic process.
11. ⇒ (AIPMT 2014 )
A monatomic gas at a pressure P, having a volume V expands isothermally to a volume 2V and then adiabatically to a volume 16V. The final pressure of the gas is (Take = 5/3)
(A) 64P
(B) 32P
(C) P/64
(D) 16P
Correct answer is (C)
First, isothermal expansion
Then, adiabatic expansion
(For adiabatic process, PV = constant)