Correct Option is (D)
16. ⇒ (MHT CET 2021 21th September Morning Shift )
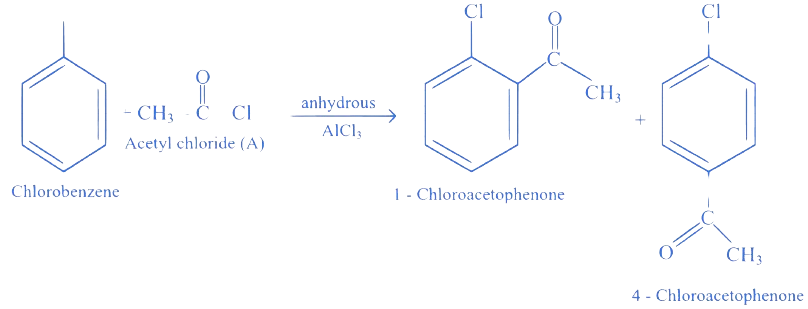
Identify reactant (A) used in the following conversion.
Chlorobenzene Chloroacetophenone Chloroacetophenone
A. Ethyl acetate
B. Acetophenone
C. Acetic acid
D. Acetyl chloride
Correct Option is (D)

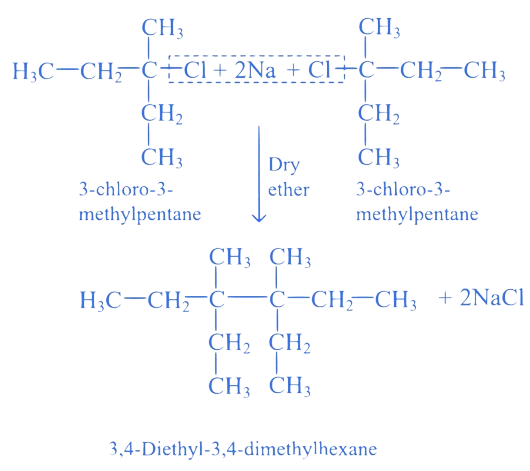
17. ⇒ (MHT CET 2021 20th September Evening Shift )
Identify '' in the following reaction.
3,4-diethyl-3,4-dimethylhexane
A. 3-Chloro-3-methylpentane
B. 3-Chloro-2-methylpentane
C. 2-Chloro-3-methylpentane
D. 2-Chioro-2-methylpentane
Correct Option is (A)
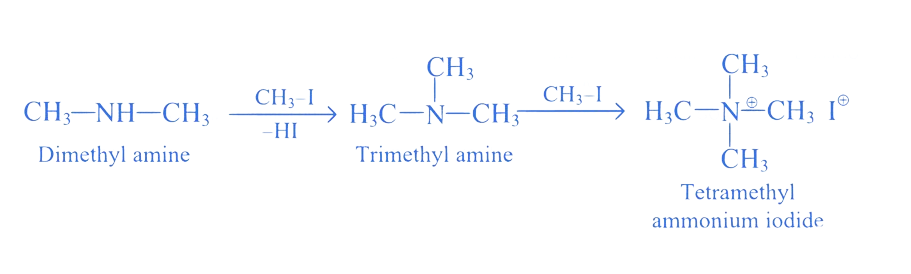
18. ⇒ (MHT CET 2021 20th September Evening Shift )
How many molecules of methyl iodide are required to obtain tetramethyl ammonium iodide from dimethyl amine?
A. 1
B. 3
C. 2
D. 4
Correct Option is (C)
19. ⇒ (MHT CET 2021 20th September Morning Shift )
Which among the following is NOT correct statement about reaction?
A. A more powerful nucleophile favours mechanism.
B. reaction proceeds via formation of carbocation intermediate.
C. reaction proceeds more rapidly in polar protic solvent.
D. The rate of mechanism is independent of the nature of nucleophile.
Correct Option is (A)
A more powerful nucleophile attacks the substrate faster and favours mechanism.