Correct answer is option (A)
displaced
Reduction and Oxidation both are taking place.
1. Which of the following options are correct for the reaction
A. Redox reaction
B. Displacement reaction
C. Decomposition reaction
D. Combination reaction
Choose the correct answer from the options given below: ⇒ (JEE Main 2023 (Online) 6th April Morning Shift)
A. A and B only
B. C and D only
C. A only
D. A and D only
Correct answer is option (A)
displaced
Reduction and Oxidation both are taking place.
2. The incorrect statement is : ⇒ (JEE Main 2021 (Online) 26th August Morning Shift )
A. Cl2 is more reactive than ClF.
B. F2 is more reactive than ClF.
C. On hydrolysis ClF forms HOCl and HF.
D. F2 is a stronger oxidizing agent than Cl2 in aqueous solution.
Correct answer is option (A)
(i) Reactivity order :
F2 > ClF (inter halogen) >
Cl2
(ii) ClF
+ H2O HOCl + HF
(iii) Oxidizing power is
aqueous
solution
F2 > Cl2 > Br2 > I2
3. The redox reaction among the following is : ⇒ (JEE Main 2020 (Online) 7th January Evening Slot)
A. reaction of H2SO4 with NaOH.
B. formation of ozone form atmosphere oxygen in the presence of sunlight.
C. combination of dinitrogen with dioxygen at 2000 K
D. reaction of [Co(H2O)6]Cl3 With AgNO3
Correct answer is option (C)
N2 + O2 2NO
during the reaction, oxidation of nitrogen take place
from 0 to 2 and reduction of oxygen take place
from 0 to –2. It means this reaction is redox
reaction.
3O2 2O3 (Non - redox reaction)
2NaOH + H2SO4 Na2SO4 + 2H2O
(neutralization reaction)
[Co(H2O)6]Cl3 + 3AgNO3
3AgCl +
[Co(H2O)6](NO3)3
Reaction of [CO(H2O)6]Cl3 with AgNO3 is
not
redox reaction. It is a precipitation reaction.
4. Which of the following reactions is an example of a redox reaction? ⇒ (JEE Main 2017 (Offline))
A. + H2O + 2HF
B. + 2H2O + 4HF
C. + O2F2 + O2
D. + PF5
Correct answer is option (C)

5. Copper becomes green when exposed to moist air for a long period. This is due to: ⇒ (JEE 12 April 2014 (Online) )
A. the formation of a layer of cupric hydroxide on the surface of copper
B. the formation of basic copper sulphate layer on the surface of the metal
C. the formation of a layer of cupric oxide on the surface of copper
D. the formation of a layer of basic carbonate of copper on the surface of copper
Correct answer is option (D)
The formation of layer of a basic carbonate of copper on the surface of copper.
When a copper vessel is exposed to moist air for a long time it develops a green layer on it
surface.
Copper corrodes by oxidation in which it reacts with oxygen in the air to form copper
oxide.
Copper oxide then combines with carbon dioxide to make copper carbonate, which gives it a
green
colour.
This process is called corrosion of copper.
The green material is a mixture of copper hydroxide (Cu(OH)2) and copper
carbonate
(CuCO3). The following is the reaction :
2Cu(s) + H2O(g) + CO2 + O2
⇒
Cu(OH)2 + CuCO3(s)
Copper (II) carbonate is a blue-green compound.
6. Which of the following is a redox reaction ? ⇒ (AIEEE 2002)
A. NaCl + KNO3 NaNO3 + KCl
B. CaC2O4 + 2HCl CaCl2 + H2C2O4
C. Mg(OH)2 + 2NH4Cl MgCl2 + 2NH4OH
D. Zn + 2AgCN 2Ag+ Zn(CN)2
Correct answer is option (D)
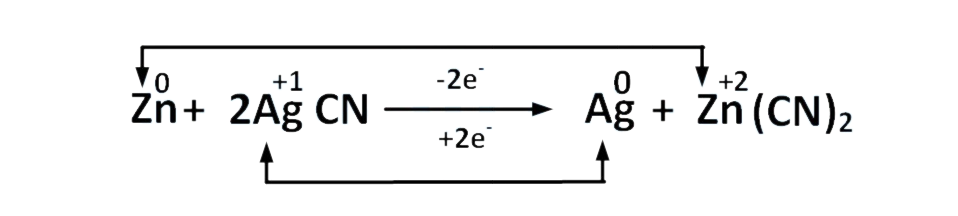
The oxidation state shows a change only in (d)