Correct option is (A)
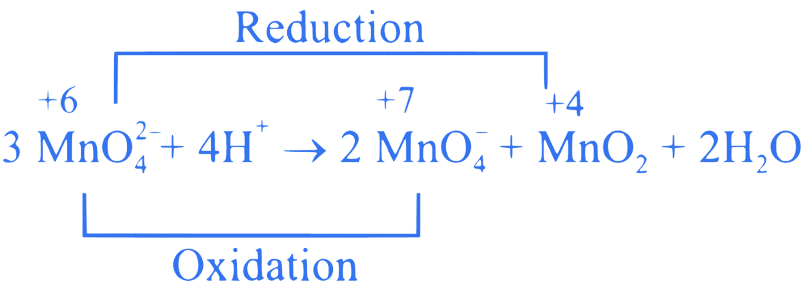
is an intermediate oxidation state and is
converted into compounds having higher and lower
oxidation states.
1. ⇒ ( JEE Main 2022 (Online) 26th June Evening Shift)
Which one of the following is an example of disproportionation reaction ?
A.
B.
C.
D.
Correct option is (A)

is an intermediate oxidation state and is
converted into compounds having higher and lower
oxidation states.
2. ⇒ (JEE Main 2019 (Online) 12th April Morning Slot )
An example of a disproportionation reaction is :
A. 2NaBr + Cl2 2NaCl + Br2
B. 2KMnO4 + MnO2 + O2 (3) (4)
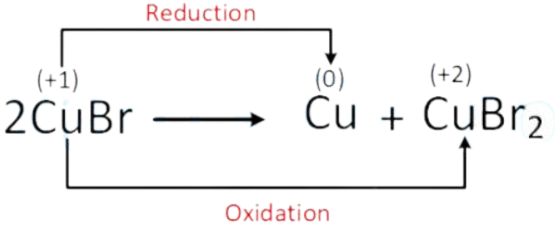
C. 2CuBr CuBr2 + Cu
D. 2MnO4 + 10I– + 16H+ 2Mn2+ + 5I2 + 8H2O
Correct option is (C)
In disproportionation reaction one element undergoes both oxidation and reduction.
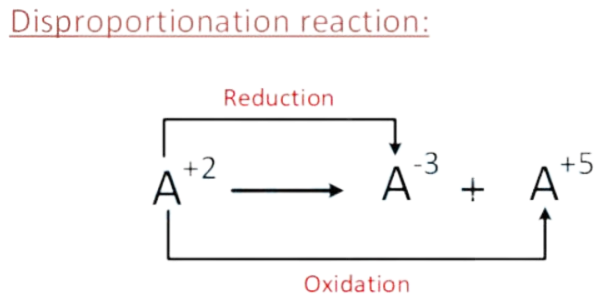
Here disproportionation reaction is :