Correct option is (A)
In H3PO4, P is present in +5 oxidation state and it can act as reducing agent only.
1. ⇒ (JEE Main 2020 (Online) 9th January Morning Slot)
The compound that cannot act both as oxidising and reducing agent is :
A. H3PO4
B. H2SO3
C. H2O2
D. HNO2
Correct option is (A)
In H3PO4, P is present in +5 oxidation state and it can act as reducing agent only.
2. ⇒ ( JEE Main 2019 (Online) 10th January Evening Slot)
In the reaction of oxalate with permanganate in acidic medium, the number of electrons involved in producing one molecule of CO2 is :
A. 10
B. 2
C. 1
D. 5
Correct option is (C)
The balanced reaction of oxalate with permanganate in acidic medium is :
In this redox reaction, the oxalate ion is oxidized to carbon dioxide , and the permanganate ion is reduced to manganese(II) ion .
The oxidation half-reaction, representing the change for the oxalate ion, is :
From this half-reaction, it's clear that each oxalate ion produces two CO2 molecules and releases two electrons in the process.
So, the number of electrons involved in producing one molecule of CO2 is :
Therefore, the correct answer is Option C: One electron is involved in the production of one molecule of CO2.
3. ⇒ ( JEE Main 2017 (Offline))
Which of the following reactions is an example of a redox reaction?
A. + H2O + 2HF
B. + 2H2O + 4HF
C. + O2F2 + O2
D. + PF5
Correct option is (C)
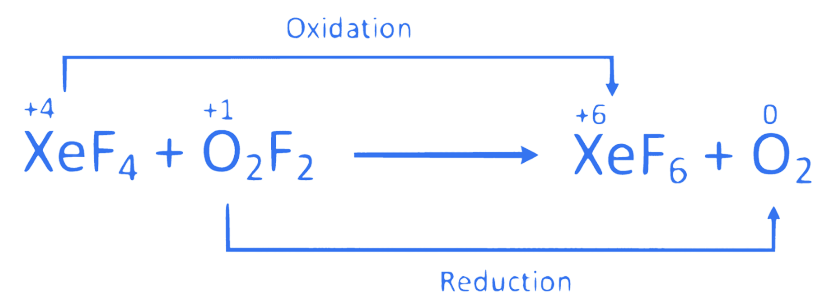
4. ⇒ (JEE Main 2014 (Offline))
In which of the following reactions H2O2 acts as a reducing agent?
1. H2O2 + 2H+ + 2e- 2H2O
2. H2O2 - 2e- O2 + 2H+
3. H2O2 + 2e- 2OH-
4. H2O2 + 2OH- - 2e- O2 + 2H2O
A. 1,2
B. 3,4
C. 1,3
D. 2,4
Correct option is (D)
The reducing agent loses electron during redox reaction i.e. oxidises itself.
5. ⇒ (AIEEE 2006)
Which of the following chemical reactions depicts the oxidizing behaviour of H2SO4?
A. NaCl + H2SO4 NaHSO4 + HCl
B. 2PCl5 + H2SO4 2POCl3 + 2HCl + SO2Cl2
C. 2HI + H2SO4 I2 + SO2 + 2H2O
D. Ca(OH)2 + H2SO4 CaSO4 + 2H2O
Correct option is (C)
in this reaction oxidation number of is decreasing from to hence undergoing reduction and for
oxidation Number of is increasing from to hence undergoing oxidation therefore
is acting as oxidising agent.