Correct option is (A)
Disproportionation
reaction are the reactions in which the same element/compound get oxidized and
reduced simultaneously.
Since I and II reactions are as follows
And
Other two reactions are correct and belong to comproportionation reaction.
Thus option A is correct.
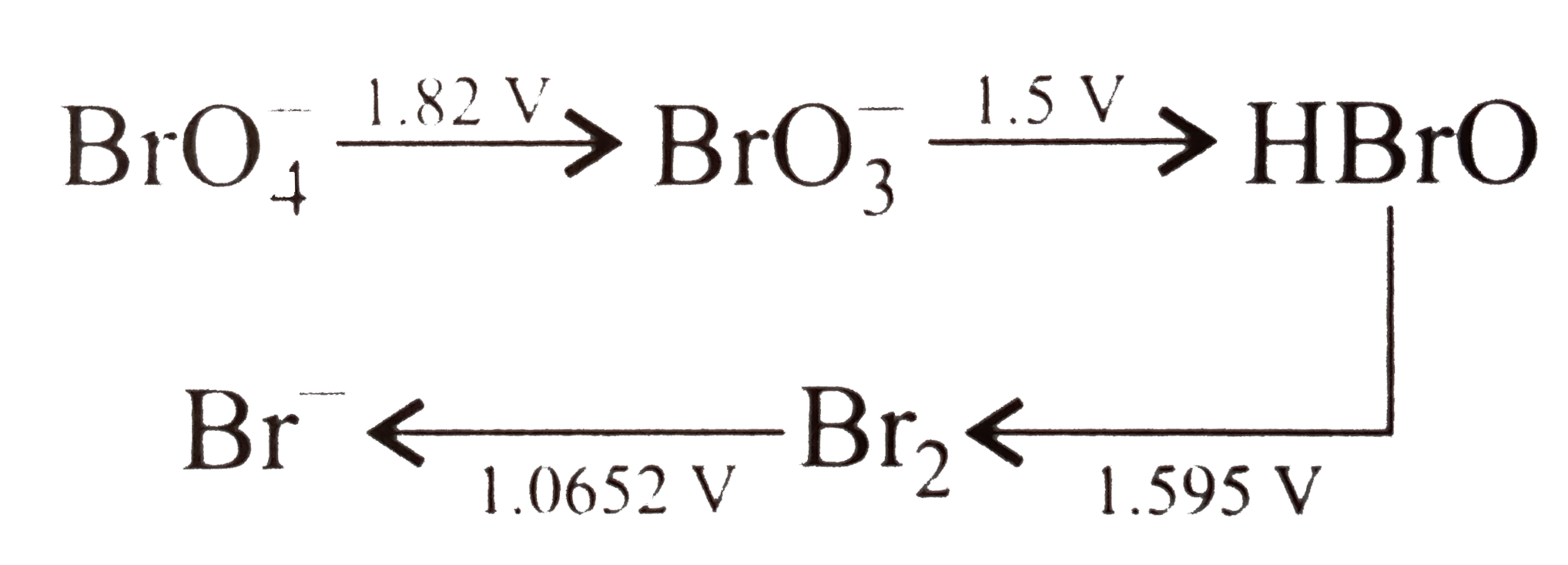 .
.