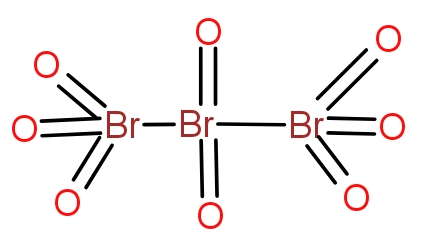
The correct answer is option (A)
The tribromo octaoxide is a neutral molecule and does not contain any charge. The structure
of
molecules
contain all oxygen atoms bonded with double bonds with bromine atoms. The molecule contains
three
bromine atoms and eight oxygen atoms.
Complete step by step answer:
Bromine
combines
with oxygen to give a variety of oxides. The tribromo octaoxide is the oxide of bromine that
contain
three bromine atoms and eight oxygen atoms. The structure of the oxide is as-
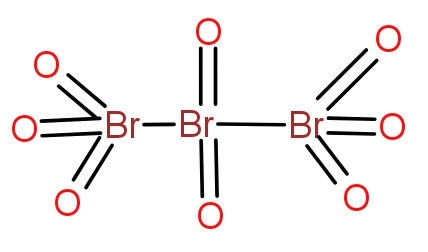
The two terminal bromine atoms bond with three oxygen atoms and a Br-Br bond. The
central
bromine
atom forms a double bond with two oxygen atoms and a single bond with two bromine atoms. The
molecule is
neutral overall and does not contain any charge.